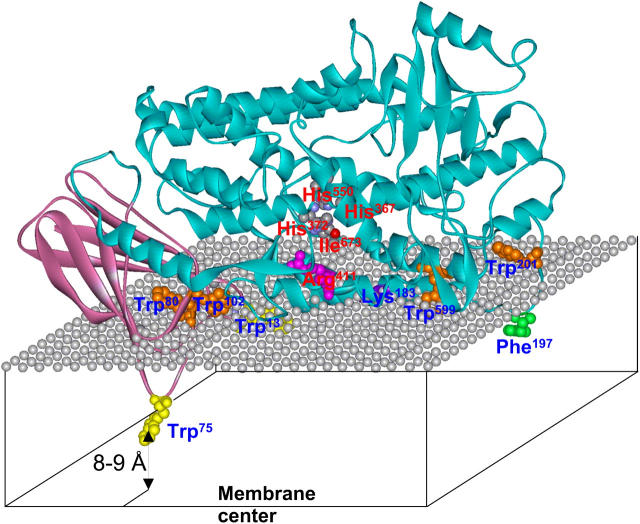
FIGURE 7.
A model for 5-LO bound to a phospholipid membrane in the fluid phase, constructed on the basis of homology modeling and data on membrane insertion and angular orientation of 5-LO. The protein structure is modeled using SWISS-MODEL on the basis of rabbit 15-LO crystal structure as a template, and is presented in a ribbon format. The N-terminal β-barrel and the catalytic domains are shown in plum and aqua, respectively. The mesh of gray spheres represents the plane of the phosphate groups of phospholipid molecules. Membrane-interacting and catalytically important residues are shown in the ball-and-stick format and are labeled by blue and red labels, respectively. Tryptophans that insert into the membrane hydrocarbon region are shown in yellow, and those located at the membrane-water interface are shown in orange. Phe197 and Lys183, which interact with the membrane by nonpolar and ionic interactions, are shown in green and purple, respectively. Arg411, which presumably forms an ionic contact with the carboxyl group of arachidonate during the initial step of enzyme-substrate interaction, is shown in magenta. Residues involved in coordination of the iron cofactor are colored according to the atom type. Trp13, and partially Trp599 and Lys183, are underneath the polar groups of lipids and can hardly be seen. His367 is hidden behind the helical ribbon. According to our earlier results (Pande et al., 2004), the symmetry axis of the N-terminal β-barrel domain is tilted at ∼45° with respect to the membrane normal, and the current data suggest that at least one Trp, most likely the Trp75, inserts into the membrane as deep as 8–9 Å from the membrane center.