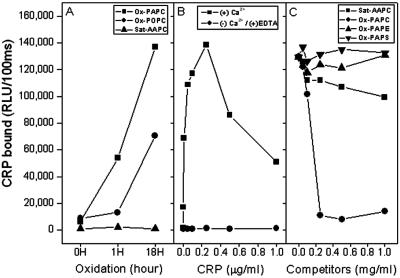
Figure 2.
Binding of CRP to PtCs and competition for the binding of CRP to PC (PC-KLH) by various phospholipids. The extent of CRP binding was determined by chemiluminescent immunoassay. (A) CRP binding to variously oxidized PtCs. Unsaturated and saturated PtC were plated in microtiter wells and air oxidized for the times indicated; CRP binding was then measured. Ox-PAPC, oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphocholine; Ox-POPC, oxidized 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; Sat-AAPC, saturated 1,2-arachidonyl-sn-glycero-3-phosphocholine (i.e., di20:0-PC). (B) Effect of calcium on the binding of CRP to oxidized PAPC. PAPC was air- oxidized in microtiter wells for 18 h, and the extent of CRP binding to oxidized PAPC was determined in the presence of calcium or in the absence of calcium and with the addition of 10 mM EDTA. (C) Competition immunoassays for binding of CRP to PC-KLH. Various phospholipids were air-oxidized for 18 h and used as competitors. Ox-PAPE, oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphatidylethanolamine; Ox-PAPS, oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphatidylserine. CRP (0.2 μg/ml in 1% BSA-TBS) was incubated in the absence or presence of indicated concentrations of phospholipids for 1 h. CRP-phospholipid complexes were pelleted by centrifugation at 1,800 × g for 30 min, and supernatants were tested for CRP-binding activity to PC-KLH. Each point is the mean of triplicate determinations.