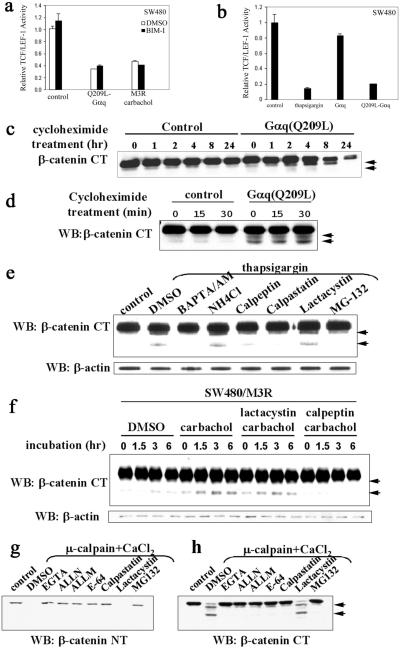
Figure 2.
Activation of the Gαq signaling pathway promotes calpain-mediated β-catenin proteolysis in a Ca2+-dependent manner. (a) The inhibitory effect of the Gq signaling pathway on TCF/LEF-1-mediated transcription is not mediated by PKC. SW480 cells were transfected with empty plasmid as control, Q209L-Gαq, or M3R expression plasmids. PKC inhibitor bisindolylmaleimide I (1 μM) was added on transfection and carbachol (1 mM) was added 24 h posttransfection. Relative TCF/LEF-1 activity was assayed 48 h after transfection. (b) Thapsigargin mimics the effect of Q209L–Gαq. SW480 cells were transfected with indicated expression plasmids. Thapsigargin (50 nM) was added on transfection. Relative TCF/LEF-1 activity was assayed 48 h after transfection. (c) Q209L–Gαq induces reduction in cytosolic β-catenin levels. SW480 cells were transfected with empty plasmid as control or Q209L–Gαq expression plasmid. Cycloheximide (25 μg/ml) was added 24 h after transfection. Cells were harvested at the indicated times after cycloheximide addition. Cytosolic fraction was prepared, resolved by SDS/PAGE, and blotted with antibody specific against the C terminus of β-catenin. Proteolytic products of β-catenin are indicated by arrowheads. (d) Q209L–Gαq induces cleavage of cytosolic β-catenin. Cytosolic fractions of SW480 cells were prepared at the indicated time points after cycloheximide treatment, electrophoretically resolved, and blotted with β-catenin antibody specific for the C terminus. Proteolytic products of β-catenin are indicated by arrowheads. (e) Thapsigargin induces calpain-mediated proteolysis of β-catenin in a calcium-dependent manner. SW480 cells were pretreated with DMSO or inhibitors for 10 min, and then treated with thapsigargin (50 nM) for 30 min. The untreated cells were used as control. The concentrations of the various inhibitors were BAPTA/AM (40 μM), NH4Cl (1 mM), calpeptin (50 μM), calpastatin (10 μM), lactacystin (10 μM), and MG-132 (10 μM). Whole-cell extract was prepared, resolved by SDS/PAGE, and blotted with the C-terminal β-catenin antibody. Proteolytic products of β-catenin are indicated by arrowheads. (f) Activation of Gq-coupled M3R induces calpain-mediated proteolysis of β-catenin. SW480 cells stably expressing M3R were treated with carbachol (1 mM), lactacystin (10 μM), or calpeptin (10 μM) as indicated. Whole-cell lysates were prepared at the indicated time points, and β-catenin fragments were detected by antibody specific for the C terminus of β-catenin. Proteolytic products of β-catenin are indicated by arrowheads. (g and h) Calpain-mediated cleavage occurs at the N-terminal region of β-catenin. As described in Materials and Methods, HEK293 cell extracts were incubated at 37°C for 30 min alone as control or with CaCl2 (0.1 mM) and μ-calpain in the presence of the indicated reagents: EGTA (1 mM), ALLN (10 μM), ALLM (10 μM), E-64 (25 μM), calpastatin (10 μM), lactacystin (10 μM), and MG-132 (10 μM). Cleavage of β-catenin was assessed with antibodies specific for the N-terminal or C-terminal regions of β-catenin. Proteolytic products of β-catenin are indicated by arrowheads. WB, Western blot; NT, N terminus; CT, C terminus.