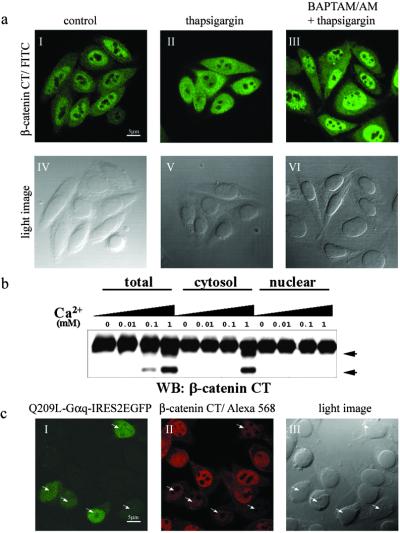
Figure 3.
Ca2+ release promotes nuclear export of β-catenin. (a) Ca2+-dependent nuclear export of β-catenin in SW480 cells. Subcellular localization of endogenous β-catenin was detected by immunofluorescence staining with anti-β-catenin (I–III). (IV–VI) Light images corresponding to I–III. (I and IV) Untreated SW480 cells as a control. (II and V) Cells that were treated with thapsigargin (50 nM) for 30 min. (III and VI) Cells that were pretreated with BAPTA/AM (50 μM) for 10 min and then incubated with thapsigargin (50 nM) for 30 min. (b) Ca2+-dependent proteolysis of β-catenin occurs in the cytoplasm. Total lysate, cytosolic, and nuclear fractions were prepared as described in Materials and Methods and incubated with the indicated amount of Ca2+ at 37°C for 30 min. β-Catenin cleavage products were detected by antibody specific for the C-terminal region after being resolved by SDS/PAGE. Proteolytic products of β-catenin are indicated by arrowheads. WB, Western blot; CT, C terminus. (c) Nuclear β-catenin is down-regulated by Q209L–Gαq. Q209L–Gαq-transfected cells were labeled by EGFP expression (I). Q209L–Gαq-expressing cells are shown by arrowheads (I–III). (Scale bars: 5 μm.)