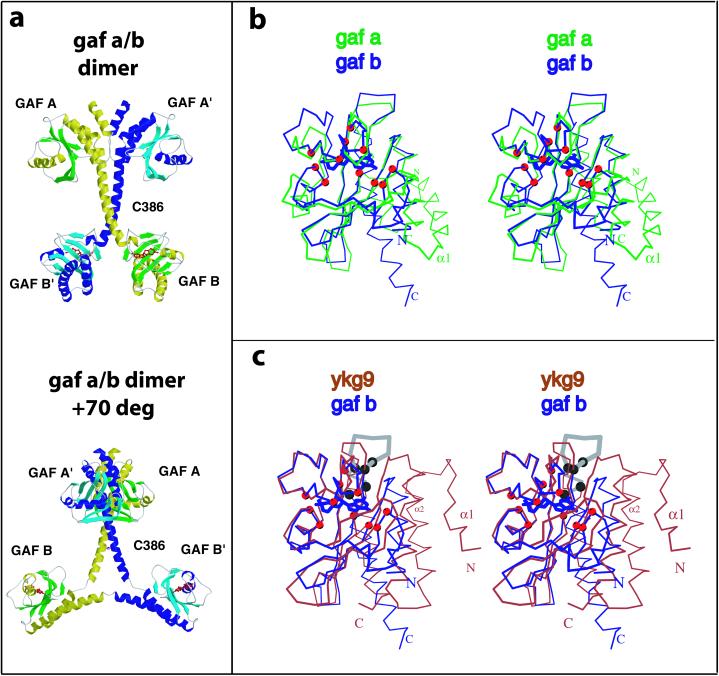
Figure 1.
(a) Two views of the structure of the regulatory segment of mouse PDE2A. Each PDE2A subunit contains a GAF A and a GAF B domain. The GAF A domain and seven turns of the connecting helices form a dimer interface. The two GAF B domains are far apart and contain the cGMP-binding sites. (Upper) View showing the dimer interface of the regulatory segment. (Lower) View ≈70° rotated with respect to A showing the Y-shape and the disulfide at C386 most clearly. cGMP is shown in red. The overall dimensions of the regulatory segment dimer are 105 × 92 × 71 Å. (b and c) Stereo images comparing GAF B with GAF A or YKG9. The Cα positions of the 11 residues that contact cGMP are shown as red spheres. (b) PDE2A GAF B (blue, with bound cGMP) and PDE2A GAF A (green) is shown after least-squares superposition of Cα positions. There are significant main-chain differences in the GAF pocket. Note that the main chain from the N terminus of helix α4 in GAF A clashes with the guanine ring of cGMP in GAF B. (c) Superposition of the Cα positions of PDE2A GAF B onto YKG9. Note that the loop in GAF B that contacts the guanine ring through D438 and F439 is turned away in YKG9. Helix α4 in GAF B is another significant difference between GAF B and YKG9. The conserved NKFDE motif predicted in early studies to be involved in cGMP binding (29) is shown in gray in GAF B. The five conserved residues in this loop (N, K, F, D, and E) are indicated by black spheres. The first four residues from the YKG9 model (monomer A, residues 4–7) point away from the domain and have been deleted for clarity.