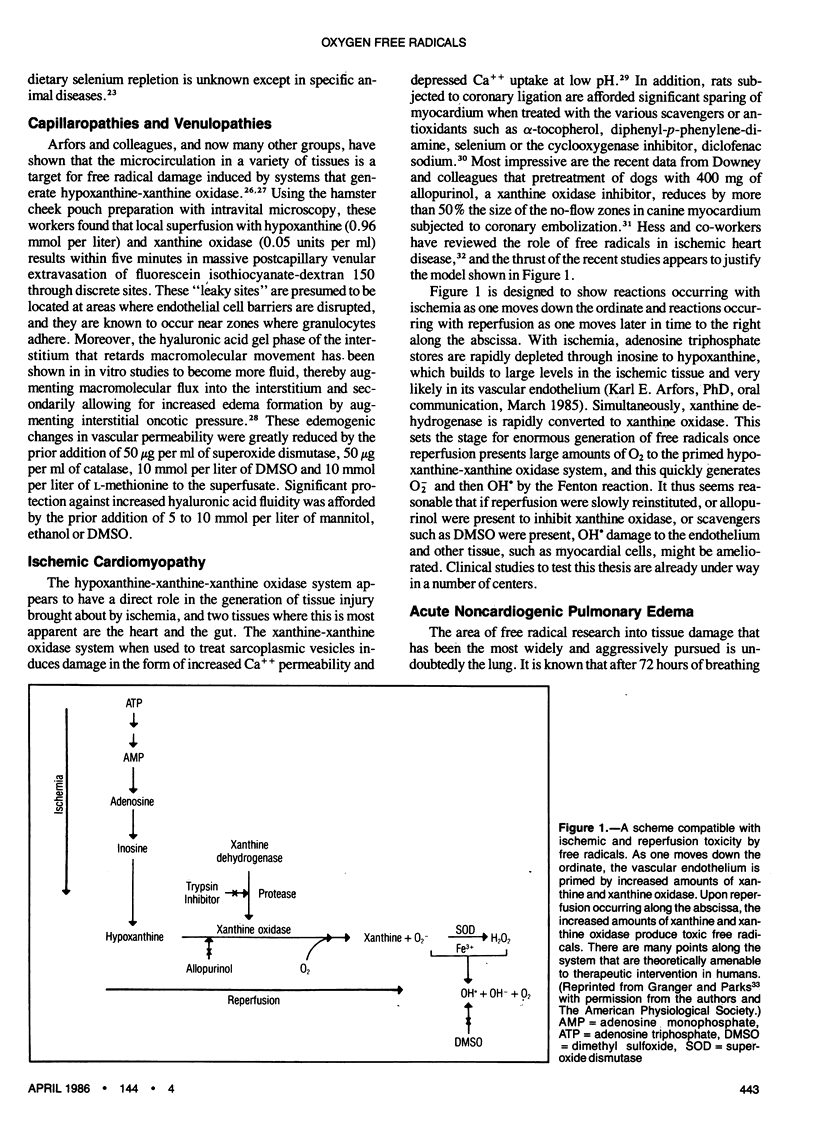
Abstract
In 1969 McCord and Fridovich discovered superoxide dismutase, which converts the oxygen free radical O2- to hydrogen peroxide H2O2. In the presence of excess O2-, H2O2 may then undergo further reduction to the highly toxic hydroxyl radical, OH•. Since the description of this enzymatic process, there has been explosive growth in related biochemical research, which has now percolated through to clinical investigation. The hypoxanthine-xanthine oxidase system originally used as a radical production model has a close counterpart in the ischemia-reperfusion phenomenon purported to cause diseases of heart, brain and gastrointestinal tract, and free radicals are now known to have a critical role in postphagocytic bacterial killing. Prototypic deficiency diseases such as chronic granulomatous disease are now recognized. Some evidence indicates that excess states such as perhaps Batten's disease also occur, and environmental influences such as selenium and vitamin E deficiency may augment free radical levels. Many disorders including microvasculopathies, noncardiogenic pulmonary edema, glomerulopathies and radiation damage may owe part of their proximate pathogenesis to free radicals. Control of tissue free radical levels is now pharmacologically feasible and perhaps justified for specific diseases.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Björk J., Arfors K. E. Oxygen free radicals and leukotriene B4 induced increase in vascular leakage is mediated by polymorphonuclear leukocytes. Agents Actions Suppl. 1982;11:63–72. [PubMed] [Google Scholar]
- Burk R. F., Lane J. M. Modification of chemical toxicity by selenium deficiency. Fundam Appl Toxicol. 1983 Jul-Aug;3(4):218–221. doi: 10.1016/s0272-0590(83)80129-8. [DOI] [PubMed] [Google Scholar]
- Burk R. F. Selenium in nutrition. World Rev Nutr Diet. 1978;30:88–106. doi: 10.1159/000401237. [DOI] [PubMed] [Google Scholar]
- Chan P. H., Schmidley J. W., Fishman R. A., Longar S. M. Brain injury, edema, and vascular permeability changes induced by oxygen-derived free radicals. Neurology. 1984 Mar;34(3):315–320. doi: 10.1212/wnl.34.3.315. [DOI] [PubMed] [Google Scholar]
- Chvapil M. Pharmacology of fibrosis and tissue injury. Environ Health Perspect. 1974 Dec;9:283–294. doi: 10.1289/ehp.749283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. Oxy-radical toxicity in catecholamine neurons. Neurotoxicology. 1984 Spring;5(1):77–82. [PubMed] [Google Scholar]
- Copeland E. S. A National Institutes of Health Workshop report. Free radicals in promotion--a chemical pathology study section workshop. Cancer Res. 1983 Nov;43(11):5631–5637. [PubMed] [Google Scholar]
- Crapo J. D., Freeman B. A., Barry B. E., Turrens J. F., Young S. L. Mechanisms of hyperoxic injury to the pulmonary microcirculation. Physiologist. 1983 Jun;26(3):170–176. [PubMed] [Google Scholar]
- Del Maestro R., Thaw H. H., Björk J., Planker M., Arfors K. E. Free radicals as mediators of tissue injury. Acta Physiol Scand Suppl. 1980;492:43–57. [PubMed] [Google Scholar]
- Demopoulos H. B., Flamm E. S., Seligman M. L., Pietronigro D. D., Tomasula J., DeCrescito V. Further studies on free-radical pathology in the major central nervous system disorders: effect of very high doses of methylprednisolone on the functional outcome, morphology, and chemistry of experimental spinal cord impact injury. Can J Physiol Pharmacol. 1982 Nov;60(11):1415–1424. doi: 10.1139/y82-210. [DOI] [PubMed] [Google Scholar]
- Edmonds P. D., Sancier K. M. Evidence for free radical production by ultrasonic cavitation in biological media. Ultrasound Med Biol. 1983 Nov-Dec;9(6):635–639. doi: 10.1016/0301-5629(83)90009-1. [DOI] [PubMed] [Google Scholar]
- Edsmyr F., Huber W., Menander K. B. Orgotein efficacy in ameliorating side effects due to radiation therapy. I. Double-blind, placebo-controlled trial in patients with bladder tumors. Curr Ther Res Clin Exp. 1976 Feb;19(2):198–211. [PubMed] [Google Scholar]
- Erdmann A. J., 3rd, Hüttemeier P. C., Landolt C., Zapol W. M. Pure oxygen breathing increases sheep lung microvascular permeability. Anesthesiology. 1983 Feb;58(2):153–158. doi: 10.1097/00000542-198302000-00009. [DOI] [PubMed] [Google Scholar]
- Fried R., Fried L. W., Babin D. R. Biological role of xanthine oxidase and tetrazolium-reductase inhibitor. Eur J Biochem. 1973 Mar 15;33(3):439–445. doi: 10.1111/j.1432-1033.1973.tb02701.x. [DOI] [PubMed] [Google Scholar]
- Gabig T. G., Kipnes R. S., Babior B. M. Solubilization of the O2(-)-forming activity responsible for the respiratory burst in human neutrophils. J Biol Chem. 1978 Oct 10;253(19):6663–6665. [PubMed] [Google Scholar]
- Granger D. N., Parks D. A. Role of oxygen radicals in the pathogenesis of intestinal ischemia. Physiologist. 1983 Jun;26(3):159–164. [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B., Westermarck T. Increased non-protein-bound iron and decreased protection against superoxide-radical damage in cerebrospinal fluid from patients with neuronal ceroid lipofuscinoses. Lancet. 1982 Aug 28;2(8296):459–460. doi: 10.1016/s0140-6736(82)90492-5. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Moody C. S. Superoxide dismutase protects against paraquat-mediated dioxygen toxicity and mutagenicity: studies in Salmonella typhimurium. Can J Physiol Pharmacol. 1982 Nov;60(11):1367–1373. doi: 10.1139/y82-204. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Eaton J. W., Balasingam M., Steinberg M. H. Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest. 1982 Dec;70(6):1253–1259. doi: 10.1172/JCI110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess M. L., Manson N. H., Okabe E. Involvement of free radicals in the pathophysiology of ischemic heart disease. Can J Physiol Pharmacol. 1982 Nov;60(11):1382–1389. doi: 10.1139/y82-206. [DOI] [PubMed] [Google Scholar]
- Hiller K. O., Hodd P. L., Willson R. L. Antiinflammatory drugs: protection of a bacterial virus as an in vitro biological measure of free radical activity. Chem Biol Interact. 1983 Dec;47(3):293–305. doi: 10.1016/0009-2797(83)90165-5. [DOI] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings L., Roholt O. A., Pressman D., Blau M., Andres G. A., Brentjens J. R. Experimental anti-alveolar basement membrane antibody-mediated pneumonitis. I. The role of increased permeability of the alveolar capillary wall induced by oxygen. J Immunol. 1981 Jul;127(1):129–134. [PubMed] [Google Scholar]
- Kontos H. A., Wei E. P., Christman C. W., Levasseur J. E., Povlishock J. T., Ellis E. F. Free oxygen radicals in cerebral vascular responses. Physiologist. 1983 Jun;26(3):165–169. [PubMed] [Google Scholar]
- Manson P. N., Anthenelli R. M., Im M. J., Bulkley G. B., Hoopes J. E. The role of oxygen-free radicals in ischemic tissue injury in island skin flaps. Ann Surg. 1983 Jul;198(1):87–90. doi: 10.1097/00000658-198307000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Noguchi T., Cantor A. H., Scott M. L. Mode of action of selenium and vitamin E in prevention of exudative diathesis in chicks. J Nutr. 1973 Oct;103(10):1502–1511. doi: 10.1093/jn/103.10.1502. [DOI] [PubMed] [Google Scholar]
- Okabe E., Hess M. L., Oyama M., Ito H. Characterization of free radical-mediated damage of canine cardiac sarcoplasmic reticulum. Arch Biochem Biophys. 1983 Aug;225(1):164–177. doi: 10.1016/0003-9861(83)90020-6. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Hoidal J. R., Ferris T. F. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984 Oct;74(4):1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor W. A. Free radical biology: xenobiotics, cancer, and aging. Ann N Y Acad Sci. 1982;393:1–22. doi: 10.1111/j.1749-6632.1982.tb31228.x. [DOI] [PubMed] [Google Scholar]
- Rehan A., Johnson K. J., Kunkel R. G., Wiggins R. C. Role of oxygen radicals in phorbol myristate acetate-induced glomerular injury. Kidney Int. 1985 Mar;27(3):503–511. doi: 10.1038/ki.1985.39. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Tate R. M. Oxygen radicals and lung edema. Physiologist. 1983 Jun;26(3):177–181. [PubMed] [Google Scholar]
- Root R. K., Rosenthal A. S., Balestra D. J. Abnormal bactericidal, metabolic, and lysosomal functions of Chediak-Higashi Syndrome leukocytes. J Clin Invest. 1972 Mar;51(3):649–665. doi: 10.1172/JCI106854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey H., Bulkley G. B., Cameron J. L. The role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Ann Surg. 1984 Oct;200(4):405–413. doi: 10.1097/00000658-198410000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. Chemical and biochemical aspects of superoxide radicals and related species of activated oxygen. Can J Physiol Pharmacol. 1982 Nov;60(11):1330–1345. doi: 10.1139/y82-200. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L. Superoxide dismutase, longevity and specific metabolic rate. Gerontology. 1982;28(4):242–244. doi: 10.1159/000212539. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Vanbenthuysen K. M., Shasby D. M., McMurtry I. F., Repine J. E. Oxygen-radical-mediated permeability edema and vasoconstriction in isolated perfused rabbit lungs. Am Rev Respir Dis. 1982 Nov;126(5):802–806. doi: 10.1164/arrd.1982.126.5.802. [DOI] [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmasoff J. M., Ono T., Cutler R. G. Superoxide dismutase: correlation with life-span and specific metabolic rate in primate species. Proc Natl Acad Sci U S A. 1980 May;77(5):2777–2781. doi: 10.1073/pnas.77.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp C., Waters B., Lebendiger G., Perkins M. Electron spin resonances of a living system (Drosophila) on normal and carcinogenic diets. Biochem Biophys Res Commun. 1983 Apr 29;112(2):602–605. doi: 10.1016/0006-291x(83)91506-1. [DOI] [PubMed] [Google Scholar]
- Van Vleet J. F., Carlton W., Olander H. J. Hepatosis dietetica and mulberry heart disease associated with selenium deficiency in Indiana swine. J Am Vet Med Assoc. 1970 Nov 1;157(9):1208–1219. [PubMed] [Google Scholar]


