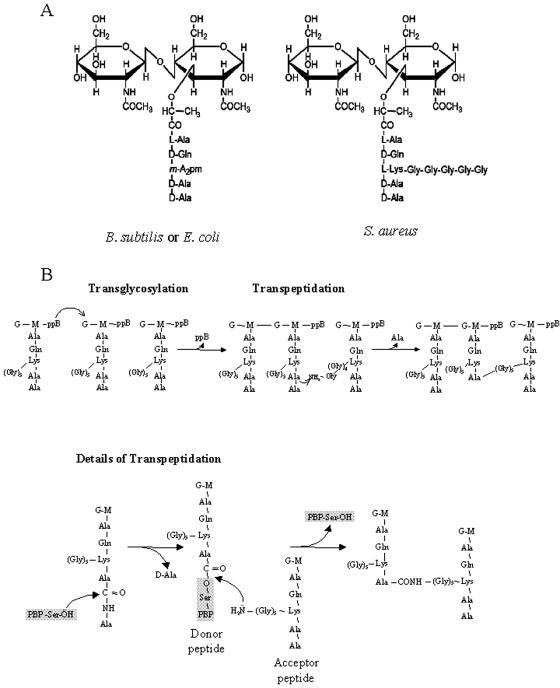
FIG. 2.
Building blocks and synthesis reactions of the peptidoglycan. (A) The basic unit of the peptidoglycan is a disaccharide-pentapeptide composed of the amino sugars N-acetylglucosamine and N-acetylmuramic acid, which are linked together by β-1,4 glycosidic bonds. The pentapeptide is covalently linked to the lactyl group of the muramic acid, and its composition can vary between different bacteria. In both E. coli and B. subtilis, the dibasic amino acid of the stem peptide, which allows the formation of the peptide cross bridge, is meso-diamoinopimelic acid (m-A2pm), while in S. aureus it is l-lysine (l-Lys), to which a pentaglycine cross bridge is bound. (B) Peptidoglycan chains are synthesized by transglycosylation and transpeptidation reactions which lead to the formation of long glycan chains cross-linked by peptide bridges. In the transglycosylation reaction, the reducing end of the N-acetylmuramic acid (M) of the nascent lipid-linked peptidoglycan strand is likely transferred onto the C-4 carbon of the N-acetylglucosamine (G) residue of the lipid-linked PG precursor, with concomitant release of undecaprenylpyrophosphate (ppB). In the transpeptidation reaction, the d-Ala-d-Ala bond of one stem peptide (donor) is first cleaved by a PBP enzyme, and an enzyme-substrate intermediate is formed, with the concomitant release of the terminal d-Ala. The peptidyl moiety is then transferred to an acceptor, which is the last amino acid of the pentaglycine cross bridge in the depicted case of S. aureus, and the PBP enzyme is released.