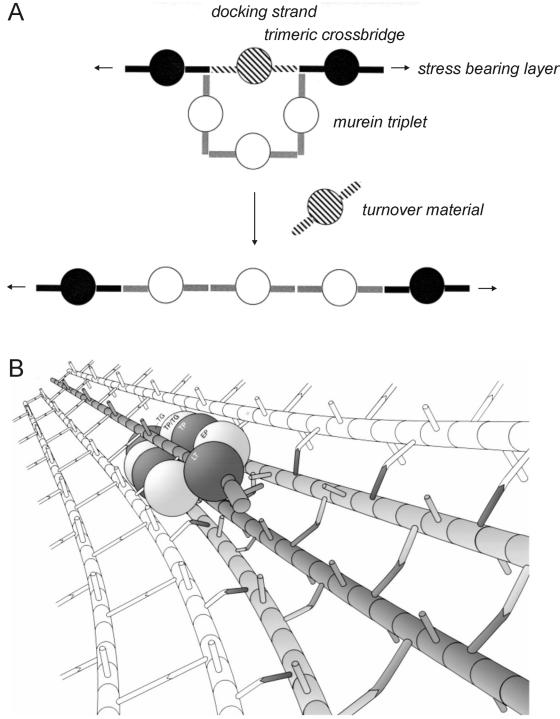
FIG. 3.
Proposed mode of insertion of new peptidoglycan in gram-negative bacteria. (A) The three-for-one growth mechanism suggests that three newly synthesized, cross-linked glycan chains in a relaxed state (white circles) are covalently attached to the free amino groups present in the donor peptides of the cross-links on both sides of a strand, called the docking strand (hatched circles), which is substituted by acceptor peptides. Specific cleavage of the preexisting cross-links results in the replacement of the docking strand by the three new, cross-linked glycan chains. (Adapted from reference 82 with permission from Elsevier.) (B) It is proposed that the three new cross-linked glycan strands (shown in gray) are synthesized by a mutienzyme complex, which also attaches these strands to the cross bridges on both sides of a docking strand (single gray strand) and at the same time degrades this strand by the action of a lytic enzymes. The peptidoglycan synthases should be in front of the hydrolases, thereby acting according to the make-before-break strategy. LT, lytic transglycosylase; EP, endopeptidase; TP, transpeptidase; TP/TG, bifunctional transpeptidase-transglycosylase; TG, transglycosylase. (Adapted from reference 80 with permission.)