Abstract
The C-terminal conserved region of Plasmodium falciparum merozoite surface protein 3 (MSP3) is the trigger antigen of a protective immune response mediated by cytophilic antibodies. In an open, randomized, two-adjuvant (Montanide ISA 720, aluminum hydroxide) phase I clinical trial we evaluated the safety and immunogenicity of increasing doses of a long synthetic peptide construct spanning the conserved region of MSP3 targeted by biologically active antibodies (MSP3-LSP). Thirty-five healthy volunteers were randomized to receive three subcutaneous injections on days 0, 30, and 120. Of the 100 injections given, 10 caused severe local reactions, 62 caused transient mild to moderate local reactions, and 28 caused no reaction. On the basis of preestablished exclusion criteria, use of the Montanide formulation led to withdrawal of five volunteers after the second injection. This led to a reduction in the subsequent vaccine doses in four of the groups. No vaccine-related serious adverse events occurred throughout the trial. After the third injection, volunteers displayed a marked specific anti-MSP3-LSP antibody response (23/30 individuals, compared with 29/34 individuals for plasma from an area where malaria is endemic), an anti-native MSP3 antibody response (19/30 individuals), a T-cell-antigen-specific proliferative response (26/30 individuals), and gamma interferon production (25/30 individuals). In conclusion, the MSP3-LSP vaccine was immunogenic with both adjuvants, although it was unacceptably reactogenic when it was combined with Montanide. The potential usefulness of the candidate vaccine is supported by the induction of a strong cytophilic response (i.e., the type of anti-MSP3 antibodies involved in antibody-dependent, monocyte-mediated protective mechanisms in areas where malaria is endemic).
In view of the high morbidity and mortality rates due to Plasmodium falciparum and in view of the progression of drug resistance that threatens treatment efficacy, populations living in areas where this organism is endemic, especially children, are in great need of novel effective antimalaria control measures, such as an antimalaria vaccine. Merozoite surface protein 3 (MSP3), an antigen associated with the membrane of the free blood-stage parasite, is the first vaccine candidate selected on the basis of a mechanism found to correlate with protection in humans, i.e., the induction of cytophilic antibodies that are the key agents of antibody-dependent, monocyte-mediated inhibition of growth of the parasite. Indeed, previous studies have demonstrated that immunoglobulin G (IgG) from individuals who are immune to malaria could passively transfer immunity to naive infected recipients (37). Furthermore, IgG cooperates with monocytes in a mechanism of antibody-dependent inhibition of parasite growth (ADCI) in vitro (6, 8, 15). Protected individuals produce mostly cytophilic antimalaria antibodies (IgG1 or IgG3), whereas nonprotected subjects produce mostly IgG2 and IgM (1, 32, 40). The C-terminal region of MSP3, which is highly conserved in various P. falciparum isolates, was identified as the target of these biologically active antibodies (33, 34, 39). In an immunocompromised mouse model of P. falciparum infection, the transfer of both monocytes and anti-MSP3211-237 antibodies induced rapid clearance of parasites (3). MSP3-based vaccines have been shown to induce strong protection against P. falciparum challenge in primates (10, 11, 21). More recently, three peptides (peptides b, c, and d) of six peptides from the C-terminal region of MSP3 were used to affinity purify antibodies that possessed ADCI activity from sera obtained from a population in which malaria is endemic (39). The region of MSP3 containing these peptides was selected as a relatively small conserved region that might be useful as a vaccine. Our previous experience with synthesis of long polypeptides (>100 amino acids) showed that we could produce good-manufacturing-procedure material quickly and efficiently for clinical studies (13, 28). Long synthetic peptides (LSP) have been shown to be safe and immunogenic in preclinical (2, 5, 26, 27, 36, 44) and clinical (20, 28, 45) trials and, therefore, should be considered good candidates for a phase I trial.
MATERIALS AND METHODS
Study design.
This study was an open-labeled, dose range study designed to assess the safety and immunogenicity of four vaccine dose regimens combined with two different adjuvants. Six groups of six subjects received three injections of either 10, 30, 100, or 300 μg of peptide in Montanide ISA 720 (groups M10-10-10, M30-30-30, M100-100-100, and M300-300-300, respectively) or 30 or 100 μg of peptide in aluminum hydroxide (groups A30-30-30 and A100-100-100, respectively). Volunteers, who were males and females between 18 and 45 years old, were excluded if they had any preexisting acute or chronic medical condition or evidence of abnormality in clinical or laboratory findings, were pregnant, had a previous history of malaria, resided in an area where malaria is endemic for the previous 6 months and throughout the study, or had a positive antibody response to MSP3-LSP as determined by an enzyme-linked immunosorbent assay (ELISA). Informed consent was obtained from all volunteers before admission into the study, which was approved by the Ethical Review Board of the Faculty of Medicine, Lausanne, Switzerland, and was authorized by the Swiss Regulation Authorities. Thirty-six volunteers were enrolled in the study, and an escalating dose schedule, starting with a 10-μg MSP3-LSP dose, was used. In the absence of serious adverse effects (AEs) after the second injection, the next higher dose schedule was initiated. Apart from serious adverse events as defined by Guidelines for Good Clinical Practice (19), a local erythema that was more than 10 by 10 cm and/or an induration that was more than 5 cm in diameter was defined as an adverse effect, which led to withdrawal of patients from further immunization (unacceptable local reaction).
Peptides.
The vaccine peptide MSP3-LSP corresponded to a fully conserved region covering amino acids 181 to 276 (sequence, RKTKEYAEKAKNAYEK AKNAYQKANQAVLKAKEASSYDYILGWEFGGGVPEHKKEENMLSHL YVSSKDKENISKENDDVLDEKEEEAEETEEEELE) of the C-terminal region of MSP3 (length, 386 amino acid) from P. falciparum strain Fc27 (29). The peptide was synthesized, purified, bottled, and lyophilized by following GMP procedures (batch 00FS023; RMF Dictagene SA, Epalinges, Switzerland; Sedac-Therapeutics, Lille, France) and was tested for stability and toxicity (sterility, endotoxin content, apyrogenicity), as well as for immunogenicity in mice. In humans, seven hyperimmune sera from Ivory Coast were shown to be able to recognize the MSP3-LSP (C. Oeuvray and P. Druilhe, unpublished data). The MSP3 210-380 protein (a kind gift of M. Theisen, Statens Seruminstitut, Copenhagen, Denmark) was used as an alternative target in antibody binding assays and consisted of a recombinant MSP3 polypeptide (rMSP3) produced in Escherichia coli, corresponding to the C-terminal region of MSP3 and covering MSP3 peptides b, c, and d (33). The following four overlapping synthetic peptides spanning 86% of MSP3-LSP were used to identify immunogenic regions by proliferative assays: MSP3-a 194-217 (HERAKNAYQKANQAVLKA KEASSY), MSP3-b211-237 (AKEASSYDYILGWEFGGGVPEHKKEEN), MSP3-c 230-257 (PEHKKEENMLSHLYVSSKDKENISKENE), and MSP3-d 238-276 (MLSHLYVSSKDKENISKENDDVLDEKEEEAEETEEEELE).
Vaccine formulation and injection.
Vaccine formulations of MSP3-LSP in combination with Montanide ISA 720 and aluminum hydroxide were prepared extemporaneously as follows. (i) Peptide MSP3-LSP was resuspended in 300 μl sterile 0.9% NaCl (Bichsel AG, Interlaken, Switzerland) and mixed with 700 μl Montanide ISA-720 (batch 95041; a kind gift from SEPPIC, Paris, France), a stable water-in-oil emulsion was formed using a 10-ml syringe (by pulling and pushing 10 times) at room temperature, and injection was performed within 5 h. (ii) The peptide was dissolved in 0.6 ml 0.9% NaCl, gently mixed with 0.6 ml aluminum hydroxide [Al(OH)3; 2 mg/ml in saline; batch 281256-01; Berna, Swiss Serum and Vaccine Institute, Bern, Switzerland], and left for 15 min at room temperature for adsorption of peptide on aluminum hydroxide (99% adsorption as measured by high-performance liquid chromatography). Injection (1 ml) was performed within 5 h via the subcutaneous route in the deltoid area on alternate sides at 0, 1, and 4 months (±10 days). The subcutaneous route was chosen to obtain the optimal conditions for surveillance of reactivity.
Safety assessment.
The assessments of safety included evaluations of reactogenicity (local and systemic reactions), tolerance (effect of vaccination on basic daily activities), and biological safety (red blood cells, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, platelets, white blood cells with differential counts, potassium, sodium, aspartate aminotransferase, alanine aminotransferase, total bilirubin, alkaline phosphatase, gamma glutamyl-transferase, creatinin, glucose, and urine analysis). Blood samples (a total of 350 ml in 12 months) were collected at months 0, 1, 2, 4, 5, 9, and 12 (50 ml at each visit; samples were taken immediately before vaccine injection when the two times coincided). A complete physical examination was done at the screening visit and at months 5, 12, and 18.
After each vaccination, the intensity, duration, and severity of any local or systemic reaction were monitored immediately during the first hour and during the two following days (see Table 3). In addition to physician observations, volunteers were asked to report adverse events and temperature (using digital thermometers) for 48 h following injection. The severity of adverse events (local or systemic AEs) was defined as mild (grade 1), moderate (grade 2), severe (grade 3), or serious (resulting in any of the following outcomes: death, a life-threatening experience, hospitalization, a persistent disability or incapacity, or any AEs requiring medical or surgical intervention to prevent one of the outcomes listed in this definition). Grading of pain (intensity) at the injection site was based on a subjective evaluation score ranging from 0 to 10, as follows: 0 to 3, mild; 4 to 6, moderate; and 7 to 10, severe. Redness and induration at the injection site were graded based on the size of the lesion. For redness the following definitions were used: 0 to 40 mm, mild; >40 to 90 mm, moderate; and >100 mm, severe. For induration the definitions were: 0 to 20 mm, mild; >20 to 40 mm, moderate; and >50 mm, severe. Movement limitation and systemic reactions were defined as mild (noticeable but no interference with normal activity), moderate (interference with normal activity), or severe (significant limitation of daily life activities). Before the second injection, to reduce the risk of hypersensitivity to the vaccine, skin prick tests were performed using a solution of MSP3-LSP (10 μg/ml) in 0.9% NaCl (ALK/Abello, Horsholm, Denmark). A prick test read at 15 min that induced a wheal that was ≤3 mm in diameter was considered negative.
TABLE 3.
Incidence of local reactions associated with vaccines after each injection
| Parameter | Group M10-10-10 | Group M20-20-20 | Group M30-30-10 | Group M100-10-10 | Group A30-30-30 | Group A100-10-10 | All groups |
|---|---|---|---|---|---|---|---|
| First injection | |||||||
| No. | 6 | 6 | 6 | 5 | 6 | 6 | 35 |
| No. with no symptoms | 2 | 4 | 1 | 0 | 3 | 1 | 11 |
| No. with pain | 2 | 1 | 0 | 4 | 1 | 1 | 9 |
| No. with functional limitation | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| No. with rednessa | 1 (0) | 1 (0) | 0 (0) | 1 (0) | 3 (0) | 1 (0) | 7 (0) |
| No. with indurationb | 1 (0) | 2 (0) | 3 (0) | 3 (0) | 1 (0) | 1 (0) | 11 (0) |
| No. with local heat | 1 | 1 | 0 | 2 | 0 | 1 | 5 |
| No. withdrawnc | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Second injection | |||||||
| No. | 6 | 6 | 6 | 5 | 6 | 6 | 35 |
| No. with no symptoms | 1 | 1 | 1 | 0 | 1 | 4 | 8 |
| No. with pain | 4 | 5 | 5 | 3 | 1 | 1 | 19 |
| No. with functional limitation | 0 | 1 | 1 | 2 | 0 | 0 | 4 |
| No. with rednessa | 0 (0) | 5 (1) | 4 (0) | 3 (0) | 4 (0) | 1 (0) | 17 (1) |
| No. with indurationb | 1 | 4 (1) | 3 (2) | 4 (2) | 2 (0) | 0 (0) | 14 (5) |
| No. with local heat | 2 | 2 | 3 | 4 | 0 | 1 | 12 |
| No. withdrawnc | 0 | 1 | 2 | 2 | 0 | 0 | 5 |
| Third injection | |||||||
| No. | 6 | 5 | 4 | 3 | 6 | 6 | 30 |
| No. with no symptoms | 2 | 2 | 1 | 1 | 0 | 3 | 9 |
| No. with pain | 4 | 0 | 2 | 1 | 3 | 3 | 13 |
| No. with functional limitation | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| No. with rednessa | 3 (1) | 3 (0) | 3 (0) | 1 (0) | 5 (1) | 2 (0) | 17 (2) |
| No. with indurationb | 2 (1) | 2 | 2 (0) | 0 (0) | 4 (1) | 0 (0) | 10 (2) |
| No. with local heat | 2 | 1 | 2 | 0 | 4 | 0 | 9 |
The numbers in parentheses are the numbers of volunteers who had redness that was ≥10 cm in diameter.
The numbers in parentheses are the numbers of volunteers who had indurations that were ≥5 cm in diameter.
Number of volunteers who did not receive further injections because of unacceptable local reactions.
Assay randomization.
Since in this study an escalating dose schedule was used, volunteers were randomized in the six treatment groups in two blocks of 18 volunteers each. The 18 volunteers in the first block were randomized in the three treatment groups in strata 1 (10-μg dose) and 2 (30-μg dose), and the 18 volunteers in the second block were randomized in the three treatment groups in strata 3 (100-μg dose) and 4 (300-μg dose). Within each stratum, volunteers were matched for sex and age by using a standard procedure in the programming language SAS 8.1 for Windows by A. M. Jensen, Statens Seruminstitut, Copenhagen, Denmark.
Immunological analysis.
At months 0, 1, 2, 4, 5, and 12, the immune responses were evaluated with whole blood samples collected with citrate as an anticoagulant (Vacutainer; BD, Basel, Switzerland); fresh peripheral blood mononuclear cells (PBMC) were separated on a density gradient, washed, and used for lymphocyte proliferation assays and cytokine production. Plasma was collected after the first centrifugation.
Antibody responses.
Anti-MSP3 antibodies in plasma samples were measured by an ELISA as described previously (35). Microtiter plates (Nunc Maxisorp, Naperville, IL) were coated with 50 μl of MSP3-LSP (or rMSP3 as indicated below) at concentrations of 1 and 7 μg/ml in phosphate-buffered saline (PBS) and blocked in PBS-5% nonfat milk. Results were expressed as the ratio of the test sample optical density (1:100 dilution) to the mean optical density plus 3 standard deviations (overall range of values, 0.251 to 0.710) for 20 individual healthy blood donor plasma samples (1:100). A ratio of >1 was considered positive. Alternatively, results were expressed as titers, which were defined as the greatest dilutions at which the sample optical density was greater than the mean optical density plus 3 standard deviations for a pool of healthy blood donor plasma.
Anti-MSP3-LSP isotypes were determined by using a previously described method (35). Results were expressed as individual ratios of postimmune optical density to preimmune plasma sample optical density.
An antibody binding competition assay was performed with plasma from individuals with the highest antibody level at month 5. Increasing concentrations of MSP3-LSP in PBS-2.5% nonfat milk were incubated for 30 min at room temperature with plasma samples at a titer corresponding to 50% of the maximal anti-MSP3-LSP ELISA response (30). LSP-bound specific anti-MSP3-LSP IgG was detected as described above.
Immunofluorescence assays and immunoblotting were performed as described previously (7, 14, 42).
T-cell proliferation assay.
Fresh PBMC were distributed in sextuplicate in 96-well flat-bottom plates (3 × 105 cells/well) (28). The PBMC were stimulated with a range of concentrations of MSP3-LSP (1.2, 6, and 30 μg/ml), with MSP3 peptides a, b, c, and d (2 and 10 μg/ml), or with medium, tetanus toxoid (10 μg/ml), or phytohemagglutinin (5 μg/ml) as controls. The median count in unstimulated cultures was 318 cpm (5th and 95th percentiles, 194 and 1,156 cpm, respectively; n = 276). Cells were harvested after 140 h, including an 18- to 20-h pulse with 1 μCi of [3H]thymidine. A response was considered positive when the stimulation index (SI) was greater than or equal to the mean SI at the baseline (preimmunization) plus 3 standard deviations.
Cytokine measurements.
Gamma interferon (IFN-γ), interleukin-6 (IL-6), and IL-10 production in response to tetanus toxoid or MSP-3 LSP was measured in proliferation supernatant after 5 days, and IL-4 production was measured after 2 days. Responses to phytohemagglutinin were measured after 2 days of culture. Supernatants were evaluated by an ELISA (Elipair, Diaclone, France) by following the manufacturer's instructions. The median limits of detection of IFN-γ, IL-10, IL-4, and IL-6 were 0.74, 0.45, 0.22, and 0.66 pg/ml, respectively.
Statistical evaluation.
The statistical analysis of clinical as well as immune responses performed was descriptive and per protocol. When indicated below, data were analyzed by a nonparametric Mann-Whitney test for unpaired comparisons, a nonparametric Wilcoxon test for paired comparisons, or a Kruskal-Wallis test for multiple group comparisons. Data correlation was performed using a parametric Pearson's test.
RESULTS
Study groups.
Fifty-two volunteers were screened for eligibility, and 36 of them were enrolled and randomly assigned to the various treatment groups (Table 1). Since two volunteers in the M30-30-30 group developed a local reaction defined as unacceptable after the second injection (see Materials and Methods), subsequent vaccine doses were revised and reduced to 10 μg for groups M30-30-30 and M100-100-100 for the third and second injections, respectively (the new groups designations were M30-30-10 and M100-10-10, respectively). Group A30-30-30 was maintained unchanged, and the dose for group A100-100-100 was also reduced to 10 μg after the second injection (new group designation, A100-10-10). Group M300-300-300 was skipped and replaced by a new group of six volunteers who received 20 μg combined with Montanide ISA 720 per immunization (group M20-20-20). There was a single spontaneous dropout after the screening visit. In total, 30 volunteers received three doses of vaccine and 5 received only two doses. The demographic characteristics of the volunteers are shown in Table 2. There was no significant difference in age, sex, and results for baseline clinical safety tests (data not shown) at the time of enrollment between the various groups.
TABLE 1.
Antigen dose regimen for the six immunization groupsa
| Dose or designation | Group 1 (Montanide) | Group 2 (Montanide) | Group 3 (aluminum hydroxide) | Group 4 (Montanide) | Group 5 (aluminum hydroxide) | Group 6 (Montanide) |
|---|---|---|---|---|---|---|
| Original protocol | ||||||
| Dose 1 (μg) | 10 | 30 | 30 | 100 | 100 | 300 |
| Dose 2 (μg) | 10 | 30 | 30 | 100 | 100 | 300 |
| Dose 3 (μg) | 10 | 30 | 30 | 100 | 100 | 300 |
| Final protocol | ||||||
| Dose 1 (μg) | 10 | 30 | 30 | 100 | 100 | 20 |
| Dose 2 (μg) | 10 | 30 | 30 | 10 | 10 | 20 |
| Dose 3 (μg) | 10 | 10 | 30 | 10 | 10 | 20 |
| Designation | M10-10-10 | M30-30-10 | A30-30-30 | M100-10-10 | A100-10-10 | M20-20-20 |
Each volunteer received three doses of peptide in association with Montanide ISA 720 (1 ml of a water-oil emulsion) or aluminum hydroxide (1 mg in suspension). The original immunization groups received an increasing dose regimen.
TABLE 2.
Demographic characteristics of volunteers
| Parameter | Group M10-10-10 | Group M20-20-20 | Group M30-30-10 | Group M100-10-10 | Group A30-30-30 | Group A100-10-10 |
|---|---|---|---|---|---|---|
| No. of volunteers | ||||||
| Total | 6 | 6 | 6 | 5 | 6 | 6 |
| No. of females/no. of males | 3/3 | 3/3 | 3/3 | 3/2 | 3/3 | 3/3 |
| Age (yr) | ||||||
| Mean | 29.73 | 27.46 | 25.79 | 27.29 | 26.46 | 27.23 |
| Range | 25.6-38.7 | 19.9-42.4 | 21.1-35.2 | 19.8-38.6 | 21.1-35.9 | 20.3-41.0 |
Safety and reactogenicity.
Table 3 summarizes the adverse events recorded for the six immunization groups. No vaccine-related serious adverse events occurred during the trial. There were no anaphylactic reactions after any of the 100 injections. Most of the 100 injections performed did not produce symptoms (28/100 injections) or produced transient, mild to moderate local reactions (62/100 injections). Ten grade 3 (severe) local adverse events were observed in seven volunteers (six volunteers who received Montanide and one volunteer who received aluminum hydroxide); six of these reactions occurred after the second injection, and four occurred after the third injection. For the most part mmunization-related AEs were limited to skin reactions at the injection site. Local reactions that occurred within the first 48 h were recorded for 24 volunteers after the first injection (pain in 9/35 volunteers, redness in 7/35 volunteers, induration in 11/35 volunteers, and local heat in 5/35) and for 27 volunteers after the second immunization (pain in 19/35 volunteers, redness in 17/35 volunteers, induration in 14/35 volunteers, and local heat in 12/35 volunteers). A 7- to 11-cm (range) local erythema and/or an induration that was 4 to 11 cm in diameter was observed in five volunteers in the Montanide groups (two volunteers in group M30-30-30, two volunteers in group M100-100-100, and one volunteer in group M20-20-20). The local inflammation resolved spontaneously within 48 to 72 h. According to preestablished criteria, these five volunteers did not receive further immunizations. Finally, after the third injection, local reactions were observed in 21 volunteers (pain in 13/30 volunteers, redness in 17/30 volunteers, induration in 10/30 volunteers, and local heat in 9/30 volunteers). An unacceptable local reaction was observed within 48 h in two volunteers in groups M10-10-10 (induration, 10 by 10 cm; erythema, 10 by 10 cm) and A30-30-30 (induration and erythema of the whole injected arm circumference with 2- by 3-cm controlateral erythema) and was resolved within 5 days. Moreover, in a volunteer with a mild local reaction in the M100-10-10 group, we observed a unilateral, nonpruriginous, painless, subcutaneous nodular induration that reached a maximal diameter of 25 mm 1 month after the second injection. After the third injection, similar local nodular indurations developed in two volunteers with severe local reactions in groups M10-10-10 and A30-30-30 and in one volunteer with a mild local reaction in group M30-30-10. The nodules reached maximal diameters of 25, 8, and 25 mm, respectively, 1 month after the third injection. All indurations were still palpable at the last follow-up visit (month 18).
Systemic reactions were limited to benign manifestations. Fatigue was reported by three volunteers (in groups M10-10-10, M20-20-20, and M100-10-10) after the second injection and by one volunteer (in group A100-10-10) after the third injection. Each period of fatigue was considered probably related to vaccination and resolved spontaneously within 24 h. Complaints of drowsiness for 3 h and complaints of palpitations for 1 h were reported about 6 h after the second injection by two volunteers (in groups A100-10-10 and M100-10-10) and were considered possibly related to the vaccine. Immediate hypersensitivity tests (skin prick tests) were negative for all patients. The results of clinical safety tests remained within normal values during the 1-year follow-up, and physical examinations at 18 months did not lead to detection of any abnormal sign or symptom.
Anti-MSP3-LSP and anti-rMSP3 210-380 antibody responses.
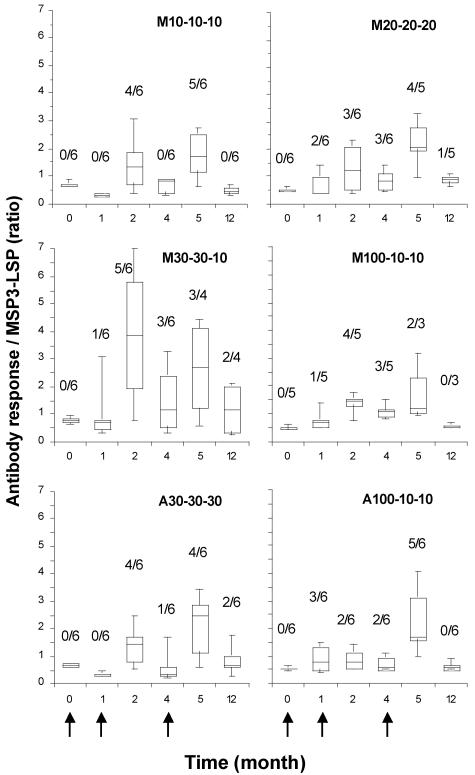
After the second and third injections, 63% (22/35) and 77% (23/30) of the volunteers, respectively, produced detectable antibodies against MSP3-LSP (ratio, >1) (Fig. 1). Following the third injection (month 5), independent of the dose and adjuvant used, the positive responses in the six groups varied from a ratio of 1.1 to a ratio of 4.5 (median, 2.7), and the titers ranged from 200 to 3,200 (median, 1,600). In comparison, 29 of 34 plasma samples (85%) from individuals living in areas where malaria is endemic were positive for anti-MSP3-LSP antibodies; the median ratio was 2.9 (range, 1.2 to 9.5), and the titers ranged from 400 to 50,000 (median, 1,600). The strongest and earliest antibody responses occurred in the M30-30-10 group (month 2); however, comparable between-group levels of responses were reached after the second and third immunizations (for between-group comparisons of A30-30-30, M30-30-10, and M20-20-20, P < 0.05). After the third injection, nonresponders were equally distributed in the different groups (4/18 volunteers in the Montanide groups and 3/12 volunteers in the aluminum hydroxide groups). Of the five volunteers who received only two injections (in groups M20-20-20, M30-30-10, and M100-10-10), four were still considered responders at month 5 and two were still considered responders at month 12.
FIG. 1.
Time course of anti-MSP3-LSP antibody response after MSP3-LSP immunization. The results are expressed as box plots and whiskers that show the 5th, 25th, 50th, 75th, and 95th percentiles for individual ratio data (optical density for the test/mean optical density for the control +3 standard deviations; plasma dilution, 1/100) for each immunization group. The arrows indicate the times of immunization. The prevalence of responders (ratio, >1) is indicated above each box.
Nineteen of 35 volunteers developed antibodies against rMSP3 210-380 (ratio, >1) at month 2, whereas 22 recognized MSP3-LSP (ratio, >1) (coefficient of determination, r2 = 0.873). Anti-rMSP3 210-380 antibody responses are shown in Table 4.
TABLE 4.
Specific anti-rMSP3 210-380 antibody responses, immunofluorescence assay results, immunoblotting results, and antibody affinity results for the six immunization groups
| Group | Anti-rMSP3 210-380 responsea
|
Immunofluorescenceb
|
Immunoblottingb: no. of responders/ total no., months 5 to 12c | Affinity: median concn (5th-95th percentiles) (g/ml), month 5d | ||||
|---|---|---|---|---|---|---|---|---|
| Median ratio (5th-95th percentiles)
|
No. of responders/total no. | Median titer (5th-95th percentiles) (dilution)
|
No. of responders/total no. | |||||
| Month 0 | Month 2 | Month 0 | Month 5 | |||||
| M10-10-10 | 0.50 (0.40-0.50) | 1.45 (0.43-3.18) | 4/6 | 38 (25-50) | 75 (31-175) | 5/6 | 3/6 | 1.50 × 10−7 (1.56 × 10−8-2.39 × 10−6) |
| M20-20-20 | 0.45 (0.40-0.50) | 1.90 (0.45-2.98) | 3/6 | 25 (25-44) | 50 (29-50) | 2/5 | 4/5 | 1.22 × 10−7 (2.48 × 10−8-2.74 × 10−7) |
| M30-30-10 | 0.45 (0.40-0.75) | 3.55 (0.65-7.20) | 5/6 | 50 (25-88) | 100 (58-185) | 3/4 | 4/4 | 7.84 × 10−9 (1.76 × 10−9-2.85 × 10−8) |
| M100-10-10 | 0.50 (0.42-0.58) | 1.10 (0.54-2.06) | 3/5 | 25 (25-25) | 25 (25-93) | 1/3 | 2/3 | 3.70 × 10−10e |
| A30-30-30 | 0.50 (0.40-0.65) | 1.00 (0.53-1.68) | 3/6 | 63 (25-325) | 150 (31-400) | 3/6 | 4/6 | 6.91 × 10−11 (5.12 × 10−11-7.62 × 10−10) |
| A100-10-10 | 0.50 (0.40-0.60) | 0.60 (0.50-1.23) | 1/6 | 25 (25-25) | 125 (31-350) | 5/6 | 5/6 | 1.08 × 10−8 (5.57 × 10−11-1.42 × 10−3) |
Anti-rMSP3 210-380 responses were measured by determining ratios as described in the text for anti-MSP3 LSP responses. A volunteer was considered a responder when the ratio was >1.
Titers in immunofluorescence assays were determined by determining the last dilution with a positive signal with P. falciparum mature schizonts. Responders were defined as individuals that presented an increase in titer after the third immunization.
Anti-native MSP3 (48 kDa) recognition was assessed by immunoblotting after incubation of plasma with P. falciparum mature schizont extract on 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels.
Affinity to MSP3-LSP was assessed by a competition assay with a plasmid positive for MSP3-LSP as determined by an ELISA. The results are expressed as soluble MSP3-LSP concentrations that inhibited antibody binding by 50%.
Data could be obtained for one of two samples.
Antibody binding competition assay.
The specific binding activity of the IgG induced by MSP3-LSP was evaluated by a competition assay using plasma from 21 volunteers with the highest antibody levels at month 5. The peptide concentrations necessary to inhibit antibody binding by 50% varied from undetectable competition in the presence of an excess of soluble peptides (in two individuals in groups M100-10-10 and A30-30-30) to concentrations as low as 36 pg/ml. Higher affinity values were observed for the aluminum hydroxide groups (median, 169 pg/ml; n = 8) than for the Montanide groups (median, 49,610 pg/ml; n = 13). The results of a detailed affinity analysis for the immunization groups are shown in Table 4.
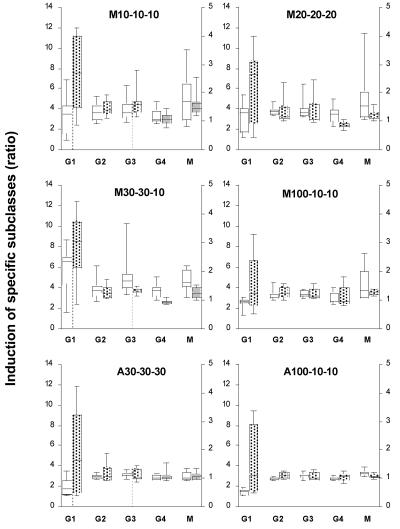
Anti-MSP3-LSP isotype antibody response.
The anti-MSP3-LSP isotype response at months 2 and 5 was predominantly an IgG1 subclass response, and it increased significantly up to the third injection (month 2 versus month 5, P < 0.001; all groups confounded) (Fig. 2). Other IgG subclass responses were lower and equally represented for the different groups (IgG2, IgG3, IgG4). As expected, IgM anti-MSP3-LSP levels tended to decrease over time (P = 0.0152). We also observed a trend toward a decrease in IgG4 in the Montanide groups after the third injection (P = 0.03), which resulted in an increase in the ratio of cytophilic to noncytophilic antibodies [(IgG1, IgG3)/(IgG2, IgG4, IgM)].
FIG. 2.
Anti-MSP3-LSP antibody isotypic responses. The results are expressed as described in the legend to Fig. 1. The responses were calculated for each isotype (IgG1 [G1], left y axis; IgG2 [G2], IgG3 [G3], IgG4 [G4], and IgM [M], right y axis) by determining the ratio of the optical density after the second injection to the preimmune optical density (open boxes) and the ratio of the optical density after the third injection to the preimmune optical density (shaded boxes) (plasma dilution, 1/100).
Recognition of the native MSP3 protein.
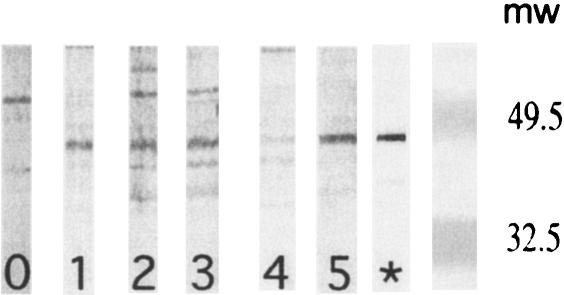
Nineteen of 30 individuals had antibodies that were reactive to native MSP3 on P. falciparum mature schizonts, as determined by immunofluorescence, at moderate titers ranging from 1/50 to 1/400 (11/18 volunteers in the Montanide groups and 8/12 volunteers in the aluminum hydroxide groups) (Table 4). We also examined the antibody reactivities of volunteers to the native parasite MSP3 protein in extracts from P. falciparum mature schizonts by immunoblotting (Fig. 3 and Table 4). Eighteen of 30 volunteers who completed the immunization schedule had antibody activity that was specific for the parasite 48-kDa protein after the third injection. Overall, 22 volunteers were positive after month 5 or month 12. The distributions of positive samples were equivalent in the different immunization groups, and noticeably, aluminum hydroxide was as effective as Montanide at eliciting parasite-reactive antibodies (13/18 volunteers in the Montanide groups and 9/12 volunteers in the aluminum hydroxide groups). There was a good correlation with the anti-MSP3-LSP-specific antibody response as determined by ELISA, since all but one volunteer with a ratio of >1.6 were positive as determined by immunoblotting.
FIG. 3.
Anti-P. falciparum recognition by immunoblotting. Plasma samples from five representative volunteers were incubated with P. falciparum mature schizont extract (3D7 clone) on 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. The lane labeled with a star contained a positive control obtained by immunizing mice with the recombinant MSP3 C-terminal protein in Montanide. Lanes 1 to 5 show representative results obtained using postimmunization samples from volunteers (month 5). They show various degrees of reactivity with the 48-kDa MSP3 protein of P. falciparum. Molecular weights (mw) (103) are indicated on the right.
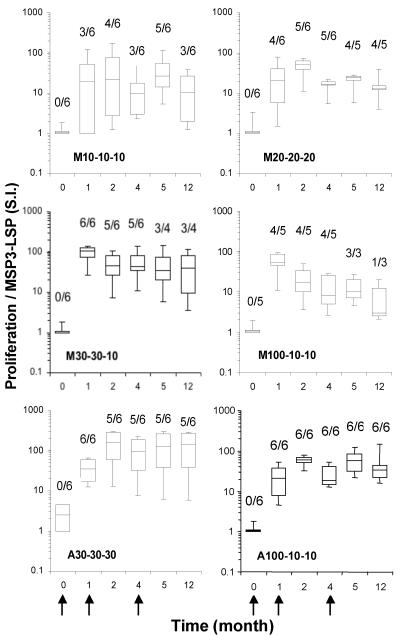
T-cell proliferative response to MSP3-LSP.
Generally, postimmunization T-cell proliferative responses were observed for all but one volunteer, and their intensities (median SI, 27; 5th percentile to 95th percentile, 1.2 to 143) were comparable to those of tetanus toxoid-stimulated cultures (median SI, 62; 5th percentile to 95th percentile, 7 to 170) (Fig. 4). The kinetics of T-cell responses to MSP3-LSP did not differ significantly in the aluminum hydroxide and Montanide groups (P < 0.05, as determined by the Kruskal-Wallis test), which displayed vigorous proliferation in most cases after priming. Immediately after the first injection, 25 (71%) volunteers developed a rapid response, which remained detectable up to month 12. Responders were equally distributed in the different groups, with minor differences. In group M10-10-10, there were only four responders after the second injection, and two volunteers developed a late response after the third injection (SI, 20 and 134). In group M20-20-20, a unique volunteer never developed a proliferative response. Two volunteers (groups M30-30-10 and A30-30-30) had a positive response after the first injection (SI, 14 and 18) that declined to the baseline 1 month later. All five volunteers who did not receive the third injection had a positive proliferative response 4 months after the second dose (SI, 6 to 98). In summary, at month 5, 26/30 volunteers developed a proliferative response. At month 12, 22/30 volunteers who completed the course of immunization and 4/5 of the volunteers excluded from the third injection still had a positive proliferative response.
FIG. 4.
Time course of anti-MSP3-LSP proliferative T-cell response. The box plots show the 5th, 25th, 50th, 75th, and 95th percentiles for the distribution of stimulation indices (S.I.) for each immunization group. SI were calculated by determining the ratio of MSP3-LSP-stimulated PBMC cultures to unstimulated PBMC cultures in cpm. Each culture was performed with six replicates. The arrows indicate the times of immunization. The prevalence of responders (SI, >3) is indicated above each box.
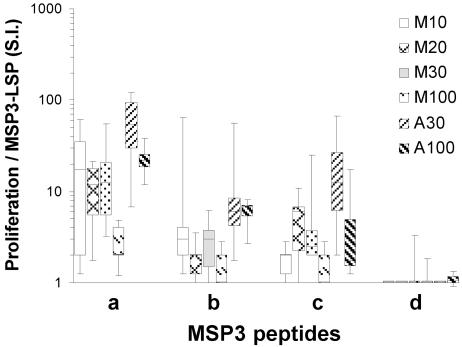
Proliferative response to MSP3 overlapping peptides.
To evaluate the epitope-specific responses, overlapping peptides designated MSP3-a, -b, -c, and -d covering amino acids 194 to 276 of MSP3-LSP were used to stimulate T cells at months 2 and 5. The T-cell responses at month 2 were high but generally lower than the responses to the full MSP3-LSP measured in parallel (P < 0.001) (Fig. 5). They were dominated by responses to fragment MSP3-a both in terms of intensity (P < 0.001) and in terms of the percentage of responders. Aluminum hydroxide clearly induced higher responses to fragments MSP3-a and -b than Montanide induced (P = 0.01 and P = 0.002, respectively). Finally, the responses to peptide MSP3-d were minimal. Data obtained at month 5 were essentially similar (data not shown).
FIG. 5.
Proliferative response to overlapping peptides MSP3-a, -b, -c, and -d. The box plots show the 5th, 25th, 50th, 75th, and 95th percentiles for the distribution of stimulation indices (S.I.) induced by the various peptides in each immunization group. SI were calculated by determining the ratio of peptide-stimulated PBMC to unstimulated PBMC in cpm after the second injection. Each culture was performed with six replicates. Stimulation with peptides MSP3-a, -b, -c, and -d was performed parallel to MSP3-LSP stimulation for comparison (Fig. 4).
Cytokine production.
Stimulation with MSP3-LSP triggered mostly IFN-γ secretion from PBMC of volunteers (median concentration, 1,080 pg/ml) (Fig. 6). Interestingly, there was parallelism between T-cell proliferative responses and IFN-γ production, since IFN-γ was detected in 30 volunteers who were also responders in the T-cell proliferation assay (i.e., in all except four volunteers). Although phytohemagglutinin induced IL-4, IL-10, and IL-6 secretion, none of the cytokines was induced by stimulation either with MSP3-LSP or with tetanus toxoid (data not shown).
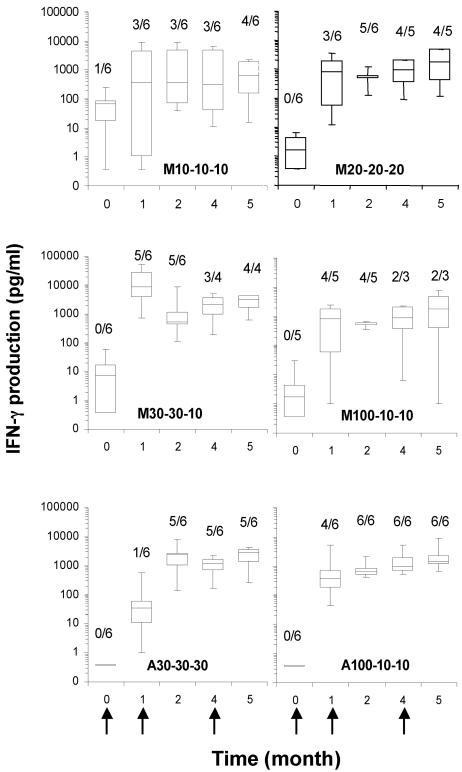
FIG. 6.
Time course of IFN-γ production by PBMC in response to MSP3-LSP. PBMC were stimulated for 5 days with 30 μg/ml MSP3-LSP. The results are expressed as box plots and whiskers that show the 5th, 25th, 50th, 75th, and 95th percentiles for individual IFN-γ secretion in the supernatant, expressed in pg/ml for each immunization group. The arrows indicate the times of immunization.
DISCUSSION
The phase I vaccine trial described here demonstrated that immunization with MSP3-LSP was able to induce vigorous specific T-cell proliferation, IFN-γ secretion, and a predominantly cytophilic IgG1 subclass humoral response, when it was administered to healthy volunteers and with both adjuvants studied. However, the Montanide formulation led to the withdrawal of an unacceptably high number of volunteers with severe local reactions, whereas the aluminum hydroxide formulation was generally well tolerated. The largely cytophilic IgG1 anti-MSP-3-specific profile of the humoral response may confer to the vaccine potential monocyte-mediated protective activity (1, 32, 40). This should be addressed in endemic area trials or with individuals exposed to malaria challenges.
In agreement with our previous observations with similar synthetic vaccine formulations derived from other antigens (20, 28, 45), MSP3-LSP was well tolerated in a majority of individuals with both formulations, and we did not observe any serious adverse events; we observed only five mild probably or possibly vaccine-related systemic reactions. Protocol-defined unacceptable reactogenicity was limited to delayed-type local inflammatory reactions within 48 h following the immunization which were more than 10 cm in diameter and/or to ≥5-cm indurations in seven volunteers. These reactions were observed in all cases except one in volunteers who received Montanide (six of seven volunteers) and led to withdrawal of five volunteers from the third injection and to marked revision of the injection doses in four of the six groups. This was justified by our policy to give priority to safety-guided decisions during the trial. Indeed, Montanide ISA 720, which is generally well tolerated when it is injected alone (24), may lead to substantial or even severe adverse events when it is combined with antigens. This may depend on the nature of the antigen tested, as shown with several P. falciparum recombinant proteins (38) or human immunodeficiency virus-derived proteins (9, 43). In all cases of withdrawal, the resolution was complete without evidence of skin ulceration, scarring, or arm function limitation, except for nodular indurations that were still palpable at month 18 in two cases for volunteers in the M10-10-10 and A30-30-30 groups. In the later trials, reactions were observed usually at high antigen doses and following the second or third injection. A review of nine previously published and unpublished vaccine trials that included 296 individuals who received vaccines with the Montanide ISA 720 adjuvant by the intramuscular route showed that there were mild to moderate local reactions in 54% of the volunteers and severe local reactions (pain, granuloma, and sterile abscesses) in 4% of the volunteers (16). There was a clear relationship between the antigen dose and the prevalence and intensity of severe, inflammatory, delayed-type local reactions (16). On the other hand, aluminum hydroxide has a well-established safety record with a low incidence of adverse events, which are limited generally to minor local reactions (4). Administered here by the subcutaneous route, MSP3-LSP with the aluminum hydroxide adjuvant induced only occasional and mild local reactions and was overall better tolerated than the MSP3-LSP associated with Montanide ISA 720.
Taken together, compared to other recent antimalaria vaccine trials in which severe hypersensitivity reactions (22, 23, 31) or marked systemic reactions (41) were reported, the reactions observed in our study were mainly local and related to the adjuvant used. In association with aluminum hydroxide, MSP3-LSP vaccine-related AEs were mild and acceptable (except in one case), in contrast to the AEs observed with the vaccine containing the Montanide adjuvant (six unacceptable local reactions). In recent trials, DNA-based vaccines induced rare local side effects, but at the cost of low immunogenicity since the humoral response after immunization was undetectable (18, 25), except after a boost with a recombinant protein vaccine (17).
A specific humoral response developed in the majority of volunteers with similar kinetics and intensity in both groups. The anti-MSP3-LSP titers compared favorably with those developed by individuals exposed to malaria in areas where malaria is endemic. The anti-MSP3-LSP response developed equally in the various groups, independent of the adjuvant used. Interestingly, the affinity of the anti-MSP3-LSP antibody response was greater in volunteers who received aluminum hydroxide than in volunteers who received Montanide. Most importantly, both adjuvants led to generation of cytophilic antibodies (mainly IgG1) (i.e., isotypes capable of supporting in vitro and in vivo antibody-dependent, monocyte-mediated parasite growth inhibition). Indeed, IgG1 and IgG3 anti-plasmodium antibodies, but not IgG2, IgG4, and IgM anti-plasmodium antibodies (1, 32) or anti-MSP3 antibodies (40), have been associated with protection in humans, as well as in primates, and to be the key players of ADCI (6, 8, 15, 33-35). We also showed that these antibodies were also able to bind a nonsynthetic recombinant MSP3 molecule (rMSP3 210-380). This fulfilled the primary end point of the trial. Moreover, native P. falciparum MSP3 protein was detectable by immunofluorescence and by immunoblotting in a substantial proportion of volunteers (19 of 30 and 22 of 30 volunteers, respectively). This is in accordance with the results of in vitro ADCI assays and in vivo SCID mouse experiments that demonstrated that vaccine-induced anti-MSP3 antibodies from sera of volunteers strongly inhibited P. falciparum development (15a).
MSP3-LSP proved able to trigger in humans a very high prevalence of strong T-cell responses, as shown by high proliferation indices and IFN-γ production in all but one volunteer. The latter point is important, since IFN-γ plays a key role in anti-blood-stage defense and, more specifically, in inducing cytophilic IgG and in enhancing the ADCI capability of monocytes (8). In addition, the T-lymphocyte memory was extremely long lasting, as shown by the high prevalence at 12 months, particularly in the aluminum hydroxide group, in which the stimulation indices remained almost unchanged. This high T-cell immunogenicity is likely related to four T-cell epitopes previously identified in humans exposed to malaria (from Senegal). In the field, peptides a, b, and c induced proliferation and IFN-γ secretion (peptide d is currently under study), with the highest prevalence for peptide b (39), in contrast to peptide a in volunteers. However, it is impossible to determine if this observation is related to human genetic differences or to differences in antigen presentation by the parasite or by the vaccine. The individuals who lost the T-cell response over time were volunteers who did not receive the third injection because of unacceptable local reactions. Globally, this indicates that both the number of injections and the dose of MSP3-LSP were important in maintaining significant proliferative and antibody responses. In agreement with the strong production of IFN-γ, the T-cell response to MSP3-LSP promoted an antibody response that was indeed largely TH1 in character (IgG1 and IgG3).
It is important to stress that the immunogenicity of MSP3-LSP formulations with aluminum hydroxide in human volunteers was not predicted by preclinical data in animal models with the same adjuvant (Druilhe, unpublished data). Aluminum hydroxide, which did not elicit detectable responses in three strains of mice, as it did in primates, appeared to be at least as efficient as Montanide in humans. The stronger immunogenicity (antibody response, T-cell proliferation, IFN-γ production) in humans may be related to our strategy of selecting the immunodominant epitopes in exposed volunteers.
Although the number of volunteers in each immunization group did not allow statistical comparison of aluminum hydroxide and Montanide, the results obtained with MSP3 with aluminum hydroxide as the adjuvant (in particular with 30 μg MSP30-LSP) in terms of immunogenicity and tolerance indicate that this adjuvant is the most valuable adjuvant for future studies. The safety record of aluminum hydroxide strongly supports this option (4, 12). A phase Ib field trial with exposed individuals and MSP3-LSP-aluminum hydroxide is currently under way.
Acknowledgments
This work was supported by a grant from European Malaria Vaccine Initiative 0003/2001CLIN.
We thank Ahmed Bouzidi for his critical contributions to GMP production; the study volunteers for their participation in the study; Géraldine Frank, Valentin Meraldi, Katrin Peter, and Alexandre Schmidt, Institute of Biochemistry, Epalinges, Switzerland, and Estelle Monnier, CHUV, Lausanne, Switzerland, for their technical assistance; A. M. Jensen, Statens Seruminstitut, Copenhagen, Denmark, for his help with assay randomization; and Stéphane Ascarateil and Jérôme Aucouturier, SEPPIC, Paris, France, for providing Montanide ISA 720.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Aribot, G., C. Rogier, J. L. Sarthou, J. F. Trape, A. T. Balde, P. Druilhe, and C. Roussilhon. 1996. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, West Africa). Am. J. Trop. Med. Hyg. 54:449-457. [DOI] [PubMed] [Google Scholar]
- 2.Astori, M., C. von Garnier, A. Kettner, N. Dufour, G. Corradin, and F. Spertini. 2000. Inducing tolerance by intranasal administration of long peptides in naive and primed CBA/J. mice. J. Immunol. 165:3497-3505. [DOI] [PubMed] [Google Scholar]
- 3.Badell, E., C. Oeuvray, A. Moreno, S. Soe, N. van Rooijen, A. Bouzidi, and P. Druilhe. 2000. Human malaria in immunocompromised mice: an in vivo model to study defense mechanisms against Plasmodium falciparum. J. Exp. Med. 192:1653-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baylor, N. W., W. Egan, and P. Richman. 2002. Aluminum salts in vaccines—US perspective. Vaccine 20:S18-S23. [DOI] [PubMed] [Google Scholar]
- 5.Blum-Tirouvanziam, U., C. Beghdadi-Rais, M. A. Roggero, D. Valmori, S. Bertholet, C. Bron, N. Fasel, and G. Corradin. 1994. Elicitation of specific cytotoxic T cells by immunization with malaria soluble synthetic polypeptides. J. Immunol. 153:4134-4141. [PubMed] [Google Scholar]
- 6.Bouharoun-Tayoun, H., P. Attanath, A. Sabchareon, T. Chongsuphajaisiddhi, and P. Druilhe. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 172:1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouharoun-Tayoun, H., and P. Druilhe. 1992. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect. Immun. 60:1473-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouharoun-Tayoun, H., C. Oeuvray, F. Lunel, and P. Druilhe. 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182:409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cano, C. A. 1999. The multi-epitope polypeptide approach in HIV-1 vaccine development. Genet. Anal. 15:149-153. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho, L. J., S. G. Oliveira, M. Theisen, F. A. Alves, M. C. Andrade, G. M. Zanini, M. C. Brigido, C. Oeuvray, M. M. Povoa, J. A. Muniz, P. Druilhe, and C. T. Daniel-Ribeiro. 2004. Immunization of Saimiri sciureus monkeys with Plasmodium falciparum merozoite surface protein-3 and glutamate-rich protein suggests that protection is related to antibody levels. Scand. J. Immunol. 59:363-372. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho, L. J. M., F. A. Alves, C. Bianco, Jr., S. G. Oliveira, G. M. Zanini, S. Soe, P. Druilhe, M. Theisen, J. A. P. C. Muniz, and C. T. Daniel-Ribeiro. 2005. Immunization of Saimiri sciureus monkeys with a recombinant hybrid protein derived from the Plasmodium falciparum antigen glutamate-rich protein and merozoite surface protein 3 can induce partial protection with Freund and Montanide ISA720 adjuvants. Clin. Diagn. Lab. Immunol. 12:242-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clements, C. J., and E. Griffiths. 2002. The global impact of vaccines containing aluminium adjuvants. Vaccine 20:S24-S33. [DOI] [PubMed] [Google Scholar]
- 13.Demotz, S., C. Moulon, M. A. Roggero, N. Fasel, and S. Masina. 2001. Native-like, long synthetic peptides as components of sub-unit vaccines: practical and theoretical considerations for their use in humans. Mol. Immunol. 38:415-422. [DOI] [PubMed] [Google Scholar]
- 14.Druilhe, P., and S. Khusmith. 1987. Epidemiological correlation between levels of antibodies promoting merozoite phagocytosis of Plasmodium falciparum and malaria-immune status. Infect. Immun. 55:888-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Druilhe, P., and J. L. Perignon. 1994. Mechanisms of defense against P. falciparum asexual blood stages in humans. Immunol. Lett. 41:115-120. [DOI] [PubMed] [Google Scholar]
- 15a.Druilhe, P., F. Spertini, S. Soe, G. Corradin, P. Meijia, S. Sing, R. Audran, A. Bouzidi, C. Oeuvray, and C. Roussilhon. A malaria vaccine that elicits in humans antibodies able to kill Plasmodium falciparum. PLOS, J., in press. [DOI] [PMC free article] [PubMed]
- 16.Dubovsky, F., and E. Tierney. 2003. Review of local reactions with Montanide ISA720. Vaccine 21:3503-3524.14518430 [Google Scholar]
- 17.Epstein, J. E., Y. Charoenvit, K. E. Kester, R. Wang, R. Newcomer, S. Fitzpatrick, T. L. Richie, N. Tornieporth, D. G. Heppner, and C. Ockenhouse. 2004. Safety, tolerability, and antibody responses in humans after sequential immunization with a PfCSP DNA vaccine followed by the recombinant protein vaccine RTS,S/AS02A. Vaccine 22:1592-1603. [DOI] [PubMed] [Google Scholar]
- 18.Epstein, J. E., E. J. Gorak, Y. Charoenvit, R. Wang, N. Freydberg, O. Osinowo, T. L. Richie, E. L. Stoltz, F. Trespalacios, J. Nerges, J. Ng, V. Fallarme-Majam, E. Abot, L. Goh, S. Parker, S. Kumar, R. C. Hedstrom, J. Norman, R. Stout, and S. L. Hoffman. 2002. Safety, tolerability, and lack of antibody responses after administration of a PfCSP DNA malaria vaccine via needle or needle-free jet injection, and comparison of intramuscular and combination intramuscular/intradermal routes. Hum. Gene Ther. 13:1551-1560. [DOI] [PubMed] [Google Scholar]
- 19.European Medicines Agency. 1995. Guidelines for good clinical practice. ICH topic E6:CPMP/ICH/135/95. European Medicines Agency, London, United Kingdom.
- 20.Fellrath, J.-M., A. Kettner, N. Dufour, C. Frigerio, D. Schneeberger, A. Leimgruber, G. Corradin, and F. Spertini. 2003. Allergen specific T cell tolerance induction with allergen-derived long peptides: results of a phase I safety and immunogenicity trial. J Allergy Clin. Immunol. 111:854-861. [DOI] [PubMed] [Google Scholar]
- 21.Hisaeda, H., A. Saul, J. J. Reece, M. C. Kennedy, C. A. Long, L. H. Miller, and A. W. Stowers. 2002. Merozoite surface protein 3 and protection against malaria in Aotus nancymai monkeys. J. Infect. Dis. 185:657-664. [DOI] [PubMed] [Google Scholar]
- 22.Kashala, O., R. Amador, M. V. Valero, A. Moreno, A. Barbosa, B. Nickel, C. A. Daubenberger, F. Guzman, G. Pluschke, and M. E. Patarroyo. 2002. Safety, tolerability and immunogenicity of new formulations of the Plasmodium falciparum malaria peptide vaccine SPf66 combined with the immunological adjuvant QS-21. Vaccine 20:2263-2277. [DOI] [PubMed] [Google Scholar]
- 23.Keitel, W. A., K. E. Kester, R. L. Atmar, A. C. White, N. H. Bond, C. A. Holland, U. Krzych, D. R. Palmer, A. Egan, C. Diggs, W. R. Ballou, B. F. Hall, and D. Kaslow. 1999. Phase I trial of two recombinant vaccines containing the 19kd carboxy terminal fragment of Plasmodium falciparum merozoite surface protein 1 (msp-1(19)) and T helper epitopes of tetanus toxoid. Vaccine 18:531-539. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence, G. W., A. Saul, A. J. Giddy, R. Kemp, and D. Pye. 1997. Phase I trial in humans of an oil-based adjuvant, SEPPIC MONTANIDE ISA 720. Vaccine 15:176-178. [DOI] [PubMed] [Google Scholar]
- 25.Le, T. P., K. M. Coonan, R. C. Hedstrom, Y. Charoenvit, M. Sedegah, J. E. Epstein, S. Kumar, R. Wang, D. L. Doolan, J. D. Maguire, S. E. Parker, P. Hobart, J. Norman, and S. L. Hoffman. 2000. Safety, tolerability and humoral immune responses after intramuscular administration of a malaria DNA vaccine to healthy adult volunteers. Vaccine 18:1893-1901. [DOI] [PubMed] [Google Scholar]
- 26.Lopez, J. A., J. M. Gonzalez, A. Kettner, M. Arevalo-Herrera, S. Herrera, G. Corradin, and M. A. Roggero. 1997. Synthetic polypeptides corresponding to the non-repeat regions from the circumsporozoite protein of Plasmodium falciparum: recognition by human T-cells and immunogenicity in owl monkeys. Ann. Trop. Med. Parasitol. 91:253-265. [PubMed] [Google Scholar]
- 27.Lopez, J. A., M. A. Roggero, O. Duombo, J. M. Gonzalez, R. Tolle, O. Koita, M. Arevalo-Herrera, S. Herrera, and G. Corradin. 1996. Recognition of synthetic 104-mer and 102-mer peptides corresponding to N- and C-terminal nonrepeat regions of the Plasmodium falciparum circumsporozoite protein by sera from human donors. Am. J. Trop. Med. Hyg. 55:424-429. [DOI] [PubMed] [Google Scholar]
- 28.Lopez, J. A., C. Weilenman, R. Audran, M. A. Roggero, A. Bonelo, J. M. Tiercy, F. Spertini, and G. Corradin. 2001. A synthetic malaria vaccine elicits a potent CD8+ and CD4+ T lymphocyte immune response in humans. Implications for vaccination strategies. Eur. J. Immunol. 31:1989-1998. [DOI] [PubMed] [Google Scholar]
- 29.McColl, D. J., and R. F. Anders. 1997. Conservation of structural motifs and antigenic diversity in the Plasmodium falciparum merozoite surface protein-3 (MSP-3). Mol. Biochem. Parasitol. 90:21-31. [DOI] [PubMed] [Google Scholar]
- 30.Men, Y., C. Thomasin, H. P. Merkle, B. Gander, and G. Corradin. 1995. A single administration of tetanus toxoid in biodegradable microspheres elicits T cell and antibody responses similar or superior to those obtained with aluminum hydroxide. Vaccine 13:683-689. [DOI] [PubMed] [Google Scholar]
- 31.Nardin, E. H., G. A. Oliveira, J. M. Calvo-Calle, Z. R. Castro, R. S. Nussenzweig, B. Schmeckpeper, B. F. Hall, C. Diggs, S. Bodison, and R. Edelman. 2000. Synthetic malaria peptide vaccine elicits high levels of antibodies in vaccinees of defined HLA genotypes. J. Infect. Dis. 182:1486-1496. [DOI] [PubMed] [Google Scholar]
- 32.Ndungu, F. M., P. C. Bull, A. Ross, B. S. Lowe, E. Kabiru, and K. Marsh. 2002. Naturally acquired immunoglobulin (Ig)G subclass antibodies to crude asexual Plasmodium falciparum lysates: evidence for association with protection for IgG1 and disease for IgG2. Parasite Immunol. 24:77-82. [DOI] [PubMed] [Google Scholar]
- 33.Oeuvray, C., H. Bouharoun-Tayoun, H. Gras-Masse, E. Bottius, T. Kaidoh, M. Aikawa, M. C. Filgueira, A. Tartar, and P. Druilhe. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84:1594-1602. [PubMed] [Google Scholar]
- 34.Oeuvray, C., H. Bouharoun-Tayoun, H. Grass-Masse, J. P. Lepers, L. Ralamboranto, A. Tartar, and P. Druilhe. 1994. A novel merozoite surface antigen of Plasmodium falciparum (MSP-3) identified by cellular-antibody cooperative mechanism antigenicity and biological activity of antibodies. Mem. Inst. Oswaldo Cruz 89:77-80. [DOI] [PubMed] [Google Scholar]
- 35.Oeuvray, C., M. Theisen, C. Rogier, J. F. Trape, S. Jepsen, and P. Druilhe. 2000. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect. Immun. 68:2617-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roggero, M. A., C. Weilenmann, A. Bonelo, R. Audran, J. Renggli, F. Spertini, G. Corradin, and J. A. Lopez. 1999. Plasmodium falciparum CS C-terminal fragment: preclinical evaluation and phase I clinical studies. Parassitologia 41:421-424. [PubMed] [Google Scholar]
- 37.Sabchareon, A., T. Burnouf, D. Ouattara, P. Attanath, H. Bouharoun-Tayoun, P. Chantavanich, C. Foucault, T. Chongsuphajaisiddhi, and P. Druilhe. 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45:297-308. [DOI] [PubMed] [Google Scholar]
- 38.Saul, A., G. Lawrence, A. Smillie, C. M. Rzepczyk, C. Reed, D. Taylor, K. Anderson, A. Stowers, R. Kemp, A. Allworth, R. F. Anders, G. V. Brown, D. Pye, P. Schoofs, D. O. Irving, S. L. Dyer, G. C. Woodrow, W. R. Briggs, R. Reber, and D. Sturchler. 1999. Human phase I vaccine trials of 3 recombinant asexual stage malaria antigens with Montanide ISA720 adjuvant. Vaccine 17:3145-3159. [DOI] [PubMed] [Google Scholar]
- 39.Singh, S., S. Soe, J.-P. Mejia, C. Roussilhon, M. Theisen, G. Corradin, and P. Druilhe. 2004. Identification of a conserved region of Plasmodium falciparum MSP3 targeted by biologically active antibodies to improve vaccine design. J. Infect. Dis. 190:1010-1018. [DOI] [PubMed] [Google Scholar]
- 40.Soe, S., M. Theisen, C. Roussilhon, K.-S. Aye, and P. Druilhe. 2004. Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect. Immun. 72:247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoute, J. A., M. Slaoui, D. G. Heppner, P. Momin, K. E. Kester, P. Desmons, B. T. Wellde, N. Garcon, U. Krzych, and M. Marchand. 1997. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N. Engl. J. Med. 336:86-91. [DOI] [PubMed] [Google Scholar]
- 42.Theisen, M., S. Soe, C. Oeuvray, A. W. Thomas, J. Vuust, S. Danielsen, S. Jepsen, and P. Druilhe. 1998. The glutamate-rich protein (GLURP) of Plasmodium falciparum is a target for antibody-dependent monocyte-mediated inhibition of parasite growth in vitro. Infect. Immun. 66:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toledo, H., A. Baly, O. Castro, S. Resik, J. Laferte, F. Rolo, L. Navea, L. Lobaina, O. Cruz, J. Miguez, T. Serrano, B. Sierra, L. Perez, M. E. Ricardo, M. Dubed, A. L. Lubian, M. Blanco, J. C. Millan, A. Ortega, E. Iglesias, E. Penton, Z. Martin, J. Perez, M. Diaz, and C. A. Duarte. 2001. A phase I clinical trial of a multi-epitope polypeptide TAB9 combined with Montanide ISA 720 adjuvant in non-HIV-1 infected human volunteers. Vaccine 19:4328-4336. [DOI] [PubMed] [Google Scholar]
- 44.von Garnier, C., M. Astori, A. Kettner, N. Dufour, C. Heusser, G. Corradin, and F. Spertini. 2000. Allergen-derived long peptide immunotherapy down-regulates specific IgE response and protects from anaphylaxis. Eur. J. Immunol. 30:1638-1645. [DOI] [PubMed] [Google Scholar]
- 45.Weilenman, C., R. Audran, A. Bonelo, J. A. Lopez, G. Corradin, and F. Spertini. 2000. OM-174 a novel adjuvant; preliminary results of a human malaria vaccination. Allergologie 23:3. (Abstract.) [Google Scholar]