Abstract
The purpose of the present study was to evaluate pigs as a large-animal model for female genital infection with two Chlamydia trachomatis human serovar E strains. Sixteen-week-old specific-pathogen-free female pigs (gilts) were intravaginally infected with the trachoma type E reference strain Bour or the urogenital serovar E strain 468. Several conclusions can be drawn from our findings on the pathogenicity of a primary C. trachomatis genital infection in gilts. First of all, we demonstrated that the serovar E strains Bour and 468 could ascend in the genital tract of gilts. The serovar E strains could replicate in the superficial columnar cervical epithelium and in the superficial epithelial layer of the uterus, which are known to be the specific target sites for a C. trachomatis genital infection in women. Second, inflammation and pathology occurred at the replication sites. Third, the organisms could trigger a humoral immune response, as demonstrated by the presence of immunoglobulin M (IgM), IgG, and IgA in both serum and genital secretion samples. Our findings imply that the pig model might be useful for studying the pathology, pathogenesis, and immune response to a C. trachomatis infection of the genital system.
Chlamydia trachomatis continues to be the most common cause of sexually transmitted disease in developed countries. World Health Organization figures estimated that over 90 million new cases of C. trachomatis infection occur worldwide each year. C. trachomatis infection of the genital tract often results in pelvic inflammatory disease, ectopic pregnancy, chronic pelvic pain, epididymitis, and infant pneumonia (20). C. trachomatis genital tract infections may also increase the risk for human immunodeficiency virus infection (9). Differences in clinical courses of infection, resulting in either symptomatic or asymptomatic infections, are observed. Bacterial and host factors are believed to contribute to these differences in clinical courses, although further research is needed (11).
So far, no vaccine has been available, but studies that may lead to the development of a highly warranted vaccine have been performed. The first attempt to vaccinate children with a whole-cell vaccine initially resulted in protection, but the protection was short-lived. In animal models, whole-cell vaccination resulted in hypersensitivity reactions, so new strategies were devised. The first immunogenic molecule described was the major outer membrane protein, and this molecule has therefore been studied in great detail as a candidate vaccine. Even though complete protection was not obtained, reduced shedding was observed, and vaccine trials in animal models using naked DNA as a vaccine resulted in stimulation of both the humoral and the cellular immune responses, indicating progress in vaccine development (3).
A pig model of infection would be helpful both for vaccine studies and for studies on major determinants of C. trachomatis-mediated pathogenesis. Pigs are physiologically and genetically closely related to humans (1, 21), and sampling is easy in this large-animal model. Moreover, Tuggle et al. (2003) (21) produced cDNA libraries containing the majority of genes expressed in major porcine female reproductive tissues and subsequently confirmed the broad expression of many genes ubiquitously expressed in human genital tissues. Thus, the purpose of the present study was to evaluate pigs as a large-animal model for infection with C. trachomatis serovar E, which is the most prevalent serovar (11) isolated from genital tract infections.
MATERIALS AND METHODS
Chlamydia trachomatis strains and culture.
The trachoma type E strain Bour (ATCC VR-348B) (6) and a serovar E genital isolate (468) from a female patient with a symptomatic clinical course both in her and in her partner (11) were propagated in cycloheximide-treated HeLa cells by using standard techniques. Elementary bodies (EBs) were purified and titrated as described elsewhere (22).
Pig model.
Fifteen 13-week-old specific-pathogen-free (SPF) outbred female pigs (gilts) (Intervet Akzo Nobel) were randomly assigned to three groups (A to C) of five, each reared in separate isolation units. At 16 weeks of age, all groups were anesthetized by intramuscular injection of Zoletil 100 (Virbac) in 2% xylazine (VMD). Subsequently, groups A and B were infected by intravaginal injection of a 50% tissue culture infective dose (1 × 108 cells) of C. trachomatis strain 468 or strain Bour, respectively. The noninfected controls were inoculated with sucrose-phosphate transport medium (2-SP). All gilts were sacrificed at 21 days postinfection (p.i.). Clinical signs were monitored every other day, and body temperatures were measured weekly. Vaginal swab samples in 1 ml 2-SP for chlamydial isolation as well as in 1 ml phosphate-buffered saline for mucosal antibody determination (pH 7.3) were collected. Additionally, sera were taken immediately prior to the infection and on days 7, 14, and 21 p.i. Swab samples for isolation of C. trachomatis were stored at −70°C, and those for serum antibody determination were stored at −20°C. At euthanasia, gilts were examined for gross lesions. Samples of the liver, the spleen, the local lymph nodes, ovaries, oviducts, uterine horns and corpus, cervix, urethra, and vagina were imbedded in Methylcellulose medium and snap-frozen in liquid nitrogen to prepare cryostat tissue sections. Tissues were fixed in 10% phosphate-buffered formalin for histopathology. The Ghent University Ethical Commission approved the experimental design.
Chlamydial antigen detection.
Vaginal swab samples were shaken for 1 h at 4°C, centrifuged, and inoculated on HeLa cells grown on 13-mm coverslips in Chlamydia Trac bottles (International Medical). Chlamydial growth was monitored by use of an IMAGEN immunofluorescence test. Cryostat tissue sections (10 μm) were similarly stained. All slides were examined using confocal laser scanning microscopy (Radiance 2000MP; Bio-Rad). Positive cells were counted in five microscopic fields (magnification, 600×). The calculated mean was presented as a score ranging from 0 to 4. A score of 0 indicated that no antigen was present. Scores of 1 and 2 indicated means of 1 to 5 and 6 to 10 elementary bodies per five microscopic fields in the absence of inclusions, respectively. Scores of 3 and 4 represented 1 to 5 and 6 to 10 inclusion-positive cells, respectively.
Antibody determinations.
Pig sera were heat inactivated for 30 min at 56°C and were subsequently pretreated with kaolin to remove background activity (14). Genital swab samples were shaken for 10 seconds, and serial twofold dilutions of sera or genital secretion samples were assayed for determinations of antibody titers by use of enzyme-linked immunosorbent assay plates (Maxisorp; Nunc) coated with the appropriate concentration of whole C. trachomatis organisms diluted in phosphate-buffered saline (pH 7.4) as described elsewhere (23).
Histopathology.
Samples were processed, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. All slides were examined microscopically (Leitz), with the observer being unaware of the animal's infection status.
Statistical analysis.
Statistical calculations were performed by use of SPSS version 11.0, and calculations for all groups were compared using the Mann-Whitney rank sum test. A P value of ≤0.05 was considered to be significant.
RESULTS
Clinical signs and gross lesions.
The mean body temperatures of groups A (468) and B (Bour) significantly increased during the experiment compared to that of noninfected control group C. Eighty percent of the pigs of group A showed hyperemia of the vulva. The same was observed in 60% of the pigs of group B. At necropsy, severe gross lesions were observed in 60% and 20% pigs of groups A and B, respectively, and these pigs showed hyperemia of the vagina; severe congestion and edema of the uterine wall; large amounts of clear watery uterine contents; severe congestion of the upper genital tract, the mesovarium, and the urethra; and enlarged local draining lymph nodes. The remaining 40% and 80% animals of groups A and B, respectively, showed similar but less prominent lesions. Negative controls showed no significantly elevated body temperatures or other clinical signs or gross lesions.
C. trachomatis vaginal excretion.
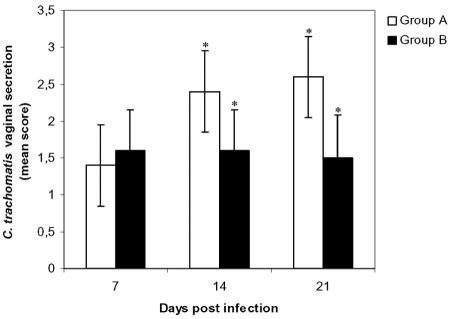
Fig. 1 shows the results of the vaginal cultures at days 7, 14, and 21 p.i. Shedding was consistently, but not extraordinarily, high for group B (Bour). Shedding was enhanced for group A (468) during the experiment, so that the median scores for vaginal shedding on day 14 (P = 0.05) and 21 (P = 0.03) p.i. were significantly higher than those for group B. Vaginal swab samples of all pigs taken before the infection were negative, and those of group C remained negative throughout the experiment.
FIG. 1.
Chlamydia trachomatis in vaginal secretion samples. Asterisks indicate statistically significant differences (P ≤ 0.05) between group A (468) and group B (Bour). Control group C remained negative throughout the experiment.
Detection in urogenital tissues.
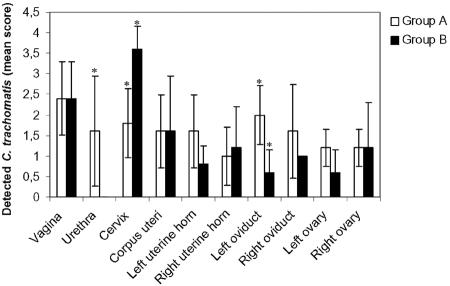
Fig. 2 shows the individual scores for the presence or replication of chlamydiae in the urogenital tract of infected gilts at 21 days p.i. All tissue samples from noninfected gilts of group C were negative, as were the spleen, the liver, and the local draining lymph nodes of groups A (468) and B (Bour). Gilts of groups A and B showed comparable, large numbers of EBs and chlamydial inclusions in the vagina. However, chlamydiae were absent in the urethra of group B, while EBs and/or urethral inclusions were observed for group A. Remarkably, the level of cervical replication for group B was significantly higher than that for group A (P = 0.007), while replication levels in the uterine corpus and uterine horns were comparable. Although EBs were present in the upper genital tract of all gilts of group B, chlamydial replication was not observed in tissues beyond the uterine corpus, except for one right uterine horn. In contrast, three of five gilts of group A (468) showed an ascending infection with chlamydial replication in the uterine horn or oviducts. In addition, the overall level of EBs in the upper genital tract was higher for group A, and for the left oviduct, a significant difference was observed (P = 0.03).
FIG. 2.
Detection of C. trachomatis in urogenital tract tissues at day 21 p.i. Asterisks indicate statistically significant differences (P < 0.05) between group A (468) and group B (Bour). Filled bars indicate replication or the presence of inclusions, while empty bars indicate the presence of EBs.
Antibody detection.
Antibody titers in sera and genital secretion samples of infected gilts of group A were present at 7 days p.i. (Table 1). For group B, serum immunoglobulin M (IgM), IgG, and IgA, as well as mucosal IgM, were also observed at 7 days p.i. Mucosal IgG and IgA in this group appeared at 14 days p.i. Serum IgM (P ≤ 0.01), IgG (P ≤ 0.01), and IgA (P ≤ 0.05 at day 7 p.i. and P ≤ 0.01 at days 14 and 21 p.i.) titers in both infected groups were always significantly different from those of the control group. The same was true for the mucosal antibody titers, except for those for IgG and IgA and for IgM, IgG, and IgA at 7 days p.i. in 468- and Bour-infected gilts, respectively. Significant differences (P ≤ 0.05) in antibody titers between both infected groups were observed only for mucosal IgG and IgA at 14 and 21 days p.i., respectively. Significant differences in serum and mucosal antibody titers within an infected group are presented in Table 1. Noninfected pigs of control group C had undetectable chlamydial antibody titers in both sera and vaginal secretion at the lowest serum dilution used (1/80).
TABLE 1.
Serum and vaginal secretion antibody titers of pigs infected with C. trachomatis strains 468 or Bour
| Strain | Day p.i. | Median titer (range from lowest to highest) of isotype-specific antibody for:
|
|||||
|---|---|---|---|---|---|---|---|
| Serum isotype
|
Mucosal isotype
|
||||||
| IgM | IgG | IgA | IgM | IgG | IgA | ||
| 468 | 7 | 2,560 (1,280-2,560)a,d | 160 (80-320)a,d | 80 (<80-320)b | 80 (<80-160)b,d | <80 (<80-80) | <80 |
| 14 | 1,280 (640-2,560)a | 1280 (640-1,280)a,e | 160 (160-320)a,d | 800 (400-1,600)a | 1,600 (800-3,200)a,c,e | 800 (400-1,600)a,d | |
| 21 | 640 (640-2,560)a | 640 (640-1,280)a,e | 160 (80-160)a,d | 800 (400-800)a | 1,600 (800-3,200)a,e | 800 (800-1,600)a,c,d | |
| Bour | 7 | 2,560 (1,280-2,560)a,d | 160 (80-320)a,d | <80 (<80-160)b | <80 (<80-80) | <80 | <80 |
| 14 | 1,280 (640-2,560)a | 640 (320-1,280)a | 160 (80-320)a,d | 800 (400-800)a | 800 (200-1,600)a,e | 400 (200-800)a,d | |
| 21 | 960 (640-2,560)a,e | 640 (320-1,280)a | 80 (80-160)a,d | 300 (200-800)a,e | 800 (200-1,600)a | 300 (200-800)a,c,d | |
P value was ≤0.01 for differences in the isotype-specific antibody titers between the infected groups and the control group.
P value was ≤0.05 for differences in the isotype-specific antibody titers between the infected groups and the control group.
P value was ≤0.05 for differences between the isotype-specific antibody titers for the 468-and Bour-infected groups.
P value was ≤0.01 for differences between isotype-specific antibody titers in serum and vaginal secretions within one group.
P value was ≤0.05 for differences between isotype-specific antibody titers in serum and vaginal secretions within one group.
Histopathology.
Histopathology (Table 2; Fig. 3) of genital specimens of group A was the most severe of the three groups, and results were comparable within the group. All gilts showed marked lesions in the vagina, which were characterized by multifocal intracellular edema together with vacuolar degeneration and cell death indicative of apoptosis (chromatin fragmentation/condensation and/or cellular shrinkage and fragmentation) of epithelial surface cells. At those specific sites, exfoliated apoptotic-like cells, neutrophils, and lymphocytes formed a siege on the epithelium. The propria showed perivascular and diffuse infiltration of neutrophils and lymphocytes. The cervix and the uterus showed few lymphocytes in the epithelium and diffuse infiltration of neutrophils, lymphocytes, and plasma cells in the submucosa. The oviducts of three of five gilts showed few lymphocytes in the lamina propria. The ovaries showed no histopathological changes. Histopathological lesions for group B were less prominent overall and differed more in severity between animals. Specimens of the negative control group C, as well as the spleen, the liver, and the local draining lymph nodes of animals of groups A and B, showed no histopathological changes.
TABLE 2.
Severities of histopathology and percent positive pigs in infected groups A and B
| Location of histopathological lesions | Description | Severity of histopathology (% positive pigs) in infected group (n = 5)a
|
|
|---|---|---|---|
| A | B | ||
| Vagina | Edema | +++ (100) | ++ (40) |
| Vacuolar degeneration | +++ (100) | ++ (40) | |
| Epithelial apoptosis | +++ (100) | ++ (40) | |
| Infiltration of neutrophils | +++ (100) | +++ (100) | |
| Infiltration of lymphocytes | +++ (100) | +++ (100) | |
| Infiltration of plasma cells | +++ (100) | +++ (100) | |
| Cervix | Congestion | +++ (100) | + (100) |
| Infiltration of neutrophils | +++ (100) | +++ (60) | |
| Infiltration of lymphocytes | +++ (100) | +++ (60) | |
| Infiltration of plasma cells | +++ (100) | +++ (60) | |
| Uterus | Congestion | +++ (100) | + (40) |
| Infiltration of neutrophils | +++ (100) | ++ (60) | |
| Infiltration of lymphocytes | +++ (100) | + (60) | |
| Infiltration of plasma cells | +++ (100) | ++ (60) | |
| Oviducts | Focal epithelial vacuolization | − (0) | + (40) |
| Infiltration of lymphocytes | + (60) | + (40) | |
| Infiltration of plasma cells | − (0) | + (40) | |
−, no lesions; +, slight lesions; ++, moderate lesions; +++, severe lesions.
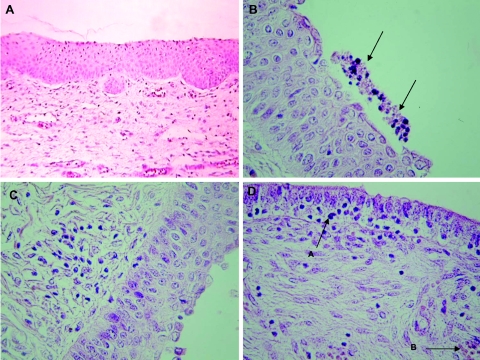
FIG. 3.
Photomicrographs of vagina, uterine corpus, and uterine horn tissue sections. All sections were stained with hematoxylin and eosin and visualized microscopically (magnification, ×400). (A) Typical uninfected vaginal morphology. (B) Inflammation of the vagina of a 468-infected gilt of group A. Note the presence of apoptotic-like cells above the epithelial layer (arrows) as well as few cells with intracellular edema in the epithelial layer. (C) Cervix of a Bour-infected gilt of group B. Note the diffuse infiltration of lymphocytes. (D) Uterine horn of a 468-infected gilt. Note the strongly folded epithelial layer with transmigrating lymphocytes and neutrophils (arrow A). The lamina propria shows focal infiltration of inflammatory cells and congestion of the blood vessels (arrow B).
DISCUSSION
The purpose of the present study was to evaluate SPF pigs as a large-animal model for female genital infection with C. trachomatis, a strict pathogen of oculogenital epithelial cells.
Current C. trachomatis animal models use the pig-tailed macaque and the mouse (10). Pigs have never been used, although pigs are physiologically and genetically highly related to humans. Pigs are susceptible hosts for Chlamydiaceae, which cause clinically unapparent infections but are also associated with a wide variety of disease syndromes, including encephalitis, conjunctivitis, enteritis, pneumonia, polyarthritis, and reproductive disorders. Pigs are naturally infected with Chlamydophila abortus, Chlamydophila pecorum, and Chlamydia suis. The latter species is phylogenetically related to human C. trachomatis strains.
Dominant C. trachomatis urogenital serovars in the Western world are D, E, and F, with serovar E being the most prevalent serovar associated with either symptomatic or asymptomatic courses of infection (13, 20, 24). Therefore, we used two serovar E strains in this study. The trachoma type E strain Bour (ATCC VR-348B) was isolated in 1959 from an adult with clinical inclusion conjunctivitis (6). Although isolated from the eye, the Bour strain caused genital infection in animal models of vaccination (15, 16). Strain 468 was isolated from a female patient and had a symptomatic clinical course of infection in both the patient and her partner (11). Strain 468 caused mucopurulent vaginal discharge and lower abdominal pain in the female patient and dysuria and mucous discharge in the male partner.
Pigs of Western origin, such as the ones used at present, have a period of antral follicular development at around 70 to 90 days of age, which is followed by a period of ovarian quiescence (17). Most follicles undergo atresia, but those whose final maturation coincides with onset of estrus will ovulate. Activation and growth of primordial follicles occur continuously throughout the life of the gilt or sow, which is necessary because the time course of follicular development is quite long. Thus, the follicle that ovulates when the gilt reaches puberty at 180 days (26 weeks) of age actually started growing more than three months earlier (2). Thus, although pigs do not have a real anestrous phase like that of mice, the age of ovarian quiescence from 13 weeks of age until 26 weeks of age can be considered as an anestrous condition. Therefore, 13-week-old SPF pigs in the anestrous phase of the estrous cycle could be used as well as sows of 26 weeks of age during the luteal phase of the estrous cycle. In the present study, we chose to use 16-week-old gilts, as 13-week-old ones may still have Chlamydiaceae-specific maternal antibodies, while 26-week-old sows are more difficult to manipulate.
No clinical signs of a C. trachomatis infection were observed, except for a reddish vulva and fever. The latter has also been described for women (4, 5). At autopsy, group A (468) showed lesions of greater severity than group B (Bour) did, although most prominent gross lesions for both groups could be observed in the cervix and the uterus of all gilts euthanized at 21 days p.i. The observed inflammation and edematous thickened cervical and uterine wall was also present in women with acute C. trachomatis salpingitis (12).
Chlamydial excretion was observed in all gilts examined at 7, 14, and 21 days p.i. At 21 days p.i., vaginal excretion for group A was significantly higher than that for group B. A possible explanation could be the additional replication of strain 468 in the urethra; strain Bour, on the other hand, was undetectable in the urethra. Strain Bour was present mainly in epithelial cells of the lower genital tract, with clear replication in the vagina and the cervix, while strain 468 was found more often in epithelial cells throughout the urogenital tract and even replicated in the oviducts. Thus, experimental inoculations resulted in an ascending infection, although strain 468 ascended faster and probably more intensely than strain Bour. However, the latter was not proven, as all animals were sacrificed at 21 days p.i. The ascending character of the infection has also been found to be present in guinea pigs, mice, and humans (11, 19). There was no replication outside the genital tract. This is in accordance with the literature stating that only lymphogranuloma venereum strains, being biologically and pathologically distinct from the strains of serovars D to K, invade the submucosa to infect macrophages and disseminate to regional lymph nodes (7).
Both serovar E strains caused acute inflammatory infiltrations in the uterus and oviducts. Uterine edema and cell vacuolization as well as oviduct cell vacuolization and hyperplasia were observed. Lesions were similar to those observed by Ramsey et al. (2000) (18) following a primary murine intravaginal infection with a C. trachomatis serovar E strain.
The infection resulted in antibodies in sera and genital secretion samples. At 14 days p.i., mucosal IgA titers, likely to be synthesized locally in the genital tract, were significantly higher than those observed in the sera. This is consistent with the observation of Kozlowski et al. (2000) (8). Mucosal antibody titers for group A were not significantly higher than those for group B.
In conclusion, we demonstrated that serovar E strains Bour and 468 could ascend in the genital tract of gilts. Both strains replicated in the superficial epithelial cervical and uterine layers, which are known to be target sites for a C. trachomatis genital infection. Inflammation and pathology occurred at the replication sites, and the organisms could trigger antibody responses. Our findings imply that the pig may be useful for studying the pathology, pathogenesis, and immune response of a C. trachomatis genital infection.
Acknowledgments
We thank L. Nabbe (Intervet Akzo Nobel) for providing the SPF gilts. We thank E. Huyck (Department of Molecular Biotechnology, UGent) and B. Mateusen (Department of Obstetrics, Reproduction and Herd Health, UGent) for their technical support. C. Puttevils (Department of Pathology, Bacteriology and Poultry Diseases, UGent) is acknowledged for assistance with graphics.
Ghent University is acknowledged for applying a research grant (011V1103).
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Bray, N., I. Dubchak, and L. A. Pachter. 2003. A global alignment program. Genome Res. 13:97-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Britt, J. H., R. A. Cushman, V. S. Hedgpeth, and D. W. Shaw. 1999. Use of an ovarian biopsy to predict surface follicle numbers on the bovine ovary. Biol. Reprod. 60(Suppl. 1):44. [Google Scholar]
- 3.Christiansen, G., and S. Birkenlund. 2002. Is a Chlamydia vaccine a reality? Best Pract. Res. Clin. Obstet. Gynaecol. 16:889-990. [DOI] [PubMed] [Google Scholar]
- 4.Eckert, L. O., S. E. Hawes, and P. K. Wolner-Hanssen. 2002. Endometritis: the clinical-pathologic syndrome. Am. J. Obstet. Gynecol. 186:690-695. [DOI] [PubMed] [Google Scholar]
- 5.Faro, S. 2001. Chlamydia trachomatis, p. 451-467. In S. Faro and D. E. Soper (ed.), Infectious diseases in women. W. B. Saunders Company, Philadelphia, Pa.
- 6.Hanna, L., P. Thygeson, and E. Jawetz. 1959. Elementary-body virus isolated from clinical trachoma in California. Science 130:1339-1340. [DOI] [PubMed] [Google Scholar]
- 7.Igietseme, J. U., C. M. Black, and H. D. Caldwell. 2002. Chlamydia vaccines: strategies and status. BioDrugs 16:19-35. [DOI] [PubMed] [Google Scholar]
- 8.Kozlowski, P. A., M. R. Neutra, T. P. Flanigan, and S. Cu-Uvin. 2000. Induction of antibodies in women after vaginal immunization during the mid-proliferative or mid-secretory phase of the menstrual cycle. FASEB J. 14(Suppl. S):A1248. [Google Scholar]
- 9.Laga, M., N. Nzila, and J. Goeman. 1991. The interrelationship of sexually transmitted diseases and HIV infection: implications for the control of both epidemics in Africa. AIDS 5(Suppl. 1):S55-S63. [PubMed] [Google Scholar]
- 10.Lichtenwalner, A. B., D. L. Patton, S. J. Klebanoff, C. M. Headley, and S. L. Hillier. 2000. Vaginal myeloperoxidase and flora in the pig-tailed macaque. J. Med. Primatol. 29:36-41. [DOI] [PubMed] [Google Scholar]
- 11.Lyons, J. M., J. I. Ito, and S. A. Morré. 2004. Chlamydia trachomatis serovar E isolates from patients with different clinical manifestations have similar courses of infection in a murine model: host factors as major determinants of C. trachomatis mediated pathogenesis. J. Clin. Pathol. 57:657-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Møller, B. R., L. Weström, S. Ahrons, K. T. Ripa, L. Svensson, C. Von Mecklenburg, H. Henrikson, and P.-A. Mårdh. 1979. Chlamydia trachomatis infection of the Fallopian tubes. Histological findings in two patients. Br. J. Vener. Dis. 55:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morré, S. A., J. M. Ossewaarde, P. H. M. Savelkoul, J. Stoof, C. J. Meijer, and A. J. Van den Brule. 2000. Analysis of genetic heterogeneity in Chlamydia trachomatis clinical isolates of serovars D, E, and F by amplified fragment length polymorphism. J. Clin. Microbiol. 38:3463-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novak, M., Z. Moldoveanu, D. P. Schafer, J. Mestecky, and R. W. Compans. 1993. Murine model for evaluation of protective immunity to influenza virus. Vaccine 11:55-60. [DOI] [PubMed] [Google Scholar]
- 15.Peterson, E. M., M. Hoshiko, B. A. Markoff, M. W. Lauermann, and L. M. de la Maza. 1990. Differences in susceptibilities of the lymphogranuloma venereum and trachoma biovars of Chlamydia trachomatis to neutralization by immune sera. Infect. Immun. 58:938-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson, E. M., J. Z. You, V. Motin, and L. M. de la Maza. 1999. Intranasal immunization with Chlamydia trachomatis, serovar E, protects from a subsequent vaginal challenge with the homologous serovar. Vaccine 17:2901-2907. [DOI] [PubMed] [Google Scholar]
- 17.Pressing, A. L., G. D. Dial, and K. E. Esbenshade. 1990. Patterns of LH secretion associated with the appearance of surface follicles in the prepubertal pig. J. Anim. Sci. 68(Suppl. 1):456. [Google Scholar]
- 18.Ramsey, K. H., J. L. DeWolfe, and R. D. Salyer. 2002. Disease outcome subsequent to primary and secondary urogenital infection with murine or human biovars of Chlamydia trachomatis. Infect. Immun. 68:7186-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rank, R. G., and M. M. Sanders. 1992. Pathogenesis of endometritis and salpingitis in a guinea pig model of chlamydial genital infection. Am. J. Pathol. 140:927-936. [PMC free article] [PubMed] [Google Scholar]
- 20.Schachter, J. 1999. Infection and disease epidemiology, p. 139-169. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 21.Tuggle, C. K., J. A. Green, C. Fitzsimmons, R. Woods, R. S. Prather, S. Malchenko, B. M. Soares, T. Kucaba, K. Crouch, C. Smith, D. Tack, N. Robinson, B. O'Leary, T. Scheetz, T. Casavant, D. Pomp, B. J. Edeal, Y. Zhang, M. F. Rothschild, K. Garwood, and W. Beavis. 2003. EST-based gene discovery in pig: virtual expression patterns and comparative mapping to human. Mamm. Genome 14:565-579. [DOI] [PubMed] [Google Scholar]
- 22.Vanrompay, D., G. Charlier, R. Ducatelle, and F. Haesebroeck. 1996. Ultrastructural changes in avian Chlamydia psittaci serovar A-, B-, and D-infected Buffalo Green Monkey cells. Infect. Immun. 64:1265-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanrompay, D., T. Geens, A. Desplanques, T. Q. T. Hoang, L. De Vos, M. Van Loock, E. Huyck, C. Mirry, and E. Cox. 2004. Immunoblotting, ELISA and culture evidence for Chlamydiaceae in sows on 258 Belgian farms. Vet. Microbiol. 99:59-66. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. 1996. Global prevalence and incidence of selected curable sexually transmitted diseases: overview and estimates. World Health Organization, Geneva, Switzerland.