Abstract
Toll-like receptor 4 (TLR4) has been shown to be important for the induction of Th2-dependent immune responses in mice. Protective immunity against larval Onchocerca volvulus in mice depends on the development of a Th2 immune response mediated by both interleukin-4 (IL-4) and IL-5. In addition, O. volvulus contains the rickettsial endosymbiont Wolbachia, which has molecules with lipopolysaccharide-like activities that also signal through TLR4. We therefore hypothesized that protective immunity to O. volvulus would not develop in C3H/HeJ mice which have a mutation in the Tlr4 gene (TLR4 mutant), either because of a decreased Th2 response to the larvae or because of the absence of a response to Wolbachia. TLR4-mutant mice were immunized against O. volvulus with irradiated third-stage larvae, and it was observed that Th2 responses were elevated based on increased IL-5 production, total immunoglobulin E (IgE) levels, antigen-specific IgG1 response, and eosinophil recruitment. Protective immunity, however, did not develop in the TLR4-mutant mice. The Th1 response, as measured by gamma interferon production from spleen cells, was comparable in both wild-type and TLR4-mutant mice. Furthermore, antibody responses to Wolbachia were absent in both wild-type and TLR4-mutant mice. Therefore, the defect in the development of a protective immune response against O. volvulus in TLR4-mutant mice is not due to loss of Th2 immunity or the response to Wolbachia but is due to an unidentified TLR4-dependent larval killing mechanism.
The filarial nematode Onchocerca volvulus is the cause of river blindness as well as a variety of other pathological sequelae, including papular dermatitis, fibrosis, and lymphadenitis in humans (16). Disease states range from mild to hyperreactive, yet there are individuals who are considered to be immunologically resistant to the infection, based on their living in an area where it is endemic and being free of infection and disease. Individual immune responses appear to be responsible for the different disease states, and roles have been ascribed for T helper 1 (Th1) cells, Th2 cells, antibodies, and granulocytes in the different disease presentations (15). Immunity against O. volvulus in putatively immune individuals has been associated with the development of a Th2 response to larval and adult antigens, with a subgroup producing a Th1 response (35). Additionally, the immune response after early exposure to O. volvulus was found to be a mixed Th1/Th2 response (8). Thus, the requirements for protective immunity in humans to the larval stages of O. volvulus remain unclear, although a Th2 response seems to be associated with the resistance status.
A mouse model was developed to study the immune response to the third-stage larvae (L3) of O. volvulus. Protective immunity was induced with irradiated L3, and the cytokines interleukin-4 (IL-4) and IL-5 were both required, thus demonstrating a dependency on a Th2 response (22). Elimination of eosinophils with monoclonal antibody (MAb) treatment at the time of the challenge infection resulted in loss of protection (2). Additionally, the development of an antibody response was required for protective immunity, and immunoglobulin E (IgE) was demonstrated to be a protective antibody isotype (2). Thus, protective immunity against O. volvulus is dependent on a Th2 immune response and is mediated by eosinophils and IgE.
Toll-like receptors (TLRs) recognize repeated patterns in pathogen-associated molecules (3). TLR4, which is activated by lipopolysaccharide (LPS) (19, 27), has been associated with the initiation of Th1 responses through the induction of proinflammatory cytokines, such as tumor necrosis factor alpha (19) and IL-12 (31). TLR4 has also been shown to be required for the development of Th2 responses. In a mouse allergic asthma model, the optimal development of a Th2 response to the allergen required signaling through TLR4 (9, 10). Additionally, TLR4 was essential for dendritic cells to direct the development of a Th2 response to a Schistosoma mansoni glycan (33). Therefore, TLR4 has varied functions during the immune response and is required for either Th1 or Th2 responses, depending on the stimulus.
O. volvulus harbors the rickettsial endosymbiont Wolbachia, found within all stages of the worm, including the L3 (4). Elimination of Wolbachia by doxycycline treatment leads to sterility of adult worms (18) and reduction of microfilarial load (17). Residents living in areas where O. volvulus is endemic have antibodies that react with Wolbachia surface protein 1 (WSP-1) (11). In addition, pathology in the eye caused by O. volvulus has been linked to the release of Wolbachia from the dying microfilariae (30). In humans, antibody responses to Brugia malayi WSP-1 were correlated with antibody responses to the L3 stage of B. malayi. Analysis of anti-WSP responses induced in mice by different stages of the rodent filaria Litomosoides sigmodontis also demonstrated that the strongest anti-WSP response was induced by the L3. It was therefore concluded that L3 were a significant source of antigenic material from Wolbachia (21). Wolbachia from O. volvulus contains molecules with LPS-like activity that induce an inflammatory response from monocytes (6). TLR4 has also been shown to be a required mediator of the ocular inflammation and pathology induced by microfilarial extracts in experimental studies in mice (14, 30). Importantly, the inflammatory response to B. malayi adult and microfilarial extracts was absent in macrophages recovered from TLR4-mutant C3H/HeJ mice (32). Furthermore, the inflammatory response elicited specifically by recombinant WSP was significantly diminished from macrophages in the absence of functional TLR4 (5). These studies therefore suggest that TLR4 is an important mediator in the response to the Wolbachia endosymbiont.
The goal of the current study was to determine the role of TLR4 in the protective immune response to O. volvulus L3. By using C3H/HeJ mice, which have a mutation in the Tlr4 gene rendering signaling through TLR4 defective (27), three hypotheses were tested in this study. First, immunity to O. volvulus L3 would be absent in TLR4-mutant mice based on the requirement of TLR4 for the development of a Th2 response. Second, immunity to O. volvulus L3 would be enhanced in TLR4-mutant mice based on the supposition that Wolbachia found in O. volvulus L3 would be recognized by the immune response and that the response induced by Wolbachia would be Th1 mediated in wild-type mice. Therefore, TLR4-mutant mice, which may lack the ability to recognize the major Wolbachia antigens, would respond to immunization with O. volvulus L3 as a purely Th2 response, thereby resulting in enhanced immunity to the infection. Third, immunity in TLR4-mutant mice would be absent, since a major target of the immune response in L3, Wolbachia, would not be recognized. It was determined, however, that in the absence of a functional TLR4 molecule, protective immunity against O. volvulus did not develop. The Th2 immune response, as measured by IL-5 production and total IgE levels, was significantly elevated in immunized TLR4-mutant mice. An anti-Wolbachia antibody response did not develop in wild-type or TLR4-mutant mice, indicating that Wolbachia is not a target of the humoral arm of the immune response in mice.
MATERIALS AND METHODS
Animals and parasites.
C3H/HeJ (TLR4-mutant) and C3H/HeOuJ (wild-type) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice were housed in filter-top microisolator boxes under light- and temperature-controlled conditions. Male mice, 6 to 8 weeks of age, were used for all experiments.
Cryopreserved L3 were prepared at the Tropical Medicine Research Station, Kumba, Cameroon, by previously described methods (2). Briefly, black flies (Simulium damnosum) were fed on consenting donors infected with O. volvulus, and after 7 days the developed L3 were collected from dissected flies, cleaned, and cryopreserved in dimethyl sulfoxide and sucrose using Biocool II computerized freezing equipment (FTS Systems Inc., Stone Ridge, NY) (34). Cryopreserved L3 were thawed by placing tubes containing the L3 on dry ice for 15 min, followed by immersion in a 37°C water bath. The L3 were then washed five times in a 1:1 mixture of Iscove's modified Dulbecco's medium and NCTC-135 with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 100 μg of gentamicin per ml, and 30 μg of chloramphenicol (Sigma Chemical Co., St. Louis, MO) per ml.
Immunization of mice with O. volvulus.
Mice were immunized in the nape of the neck with two subcutaneous injections of irradiated (35 kilorads using a cesium source) O. volvulus L3 on day 0 and day 14. The primary immunization dose consisted of 50 irradiated L3, followed 2 weeks later by a booster immunization consisting of 25 irradiated L3. On day 21, mice were challenged with 25 L3 contained within diffusion chambers implanted in a subcutaneous pocket on the rear flank of the mice. Implanted diffusion chambers were recovered on day 42, and live larvae were counted. Viability of larvae was determined by motility and morphology. Cells found within diffusion chambers were counted and centrifuged onto slides using a Cytospin 3 (Shandon, Pittsburgh, PA) and then stained for differential counts with DiffQuik (Baxter Healthcare, Miami, FL).
Construction of diffusion chambers followed previously described methods (1). Briefly, 14-mm lucite (Millipore, Bedford, MA) rings were covered with 5.0-μm-pore-size Durapore membranes (Millipore). The membranes were attached to the rings and the lucite rings were cemented to each other with a mixture consisting of equal parts of 1,2-dichloroethane (Fisher Scientific, Pittsburgh, PA) and acryloid resin (Rohm and Haas, Philadelphia, PA); the completed diffusion chambers were sterilized for 12 h in 100% ethylene oxide.
Soluble L3 and adult female antigen preparation.
Larvae, recovered after 1 or 2 days in culture, and adult female worms, obtained at the Tropical Medicine Research Station, Kumba, Cameroon, were quick-frozen in liquid nitrogen. Soluble antigens were prepared as previously described (35). Briefly, the worms were ground to a powder using a Bessman tissue pulverizer (Spectrum Lab Products, Houston, Tex.) and further disrupted by sonication before extraction in phosphate-buffered saline (PBS) containing 10 mM 3-(3-cholamidopropyl)-dimethylammonio-2-hydroxy-1-propanesulfonate (Calbiochem, San Diego, Calif.) and protease inhibitors (2 mM phenylmethylsulfonyl fluoride, 0.2 mM N-α-p-tosyl-l-lysine chloromethyl ketone, 0.2 nM N-tosyl-l-phenylalanine chloromethyl ketone, and 25 μg of leupeptin/ml, 10 mM EDTA; Sigma, St. Louis, Mo.). The insoluble material was extracted twice in the same buffer for 12 h at 4°C. The pooled soluble extracts of each stage-specific preparation were then dialyzed against PBS, centrifuged at 4°C, and filter sterilized.
Spleen cell stimulation.
Spleens from naïve and immunized mice were aseptically removed 1 week after recovery of the challenge infections and made into single-cell suspensions. Cells were cultured in a 96-well plate at 2 × 106/well. Spleen cells were stimulated with medium, soluble L3 antigens, or anti-CD3 MAb (Pharmingen, San Diego, CA). Soluble O. volvulus antigens were added for a final concentration of 1 μg/ml. Plates were coated with anti-CD3 MAb at 0.5 μg/ml for 2 h at 37°C and washed with PBS before the cells were added. Cells were incubated at 37°C for 3 days; supernatants were collected and frozen at −20°C.
Enzyme-linked immunosorbent assay (ELISA).
Measurements of serum levels of IgE used a monoclonal rat anti-mouse IgE as the capture antibody and biotinylated rat anti-mouse IgE (Pharmingen, San Diego, CA) as the detection antibody. Measurements of antigen-specific IgG responses used adult female soluble antigen on the plates and biotinylated rat anti-mouse IgG1, IgG2a, IgG2b, and IgG3 (Pharmingen) as detection antibodies. Plates were washed, and avidin peroxidase (Sigma) was added for 30 min at room temperature, followed by the peroxidase substrate ABTS [2,2′-azinodi(3-ethylbenzthiazoline-6-sulfonate); Kirkegaard & Perry Laboratories, Gaithersburg, MD]. The ABTS color reaction was measured at 410 nm on a Dynatech MR5000 microplate reader (Dynatech, Chantilly, VA).
Cytokine ELISAs for IL-5 and IL-4 were done using appropriately matched anti-IL-4 and anti-IL-5 monoclonal antibodies (Pharmingen) for coating and capture antibody. ABTS was used as the substrate. A gamma interferon (IFN-γ) ELISA kit (AN-18 monoclonal; Pharmingen) was used following the manufacturer's protocol. 3,3′,5,5′-Tetramethylbenzidine was used as the substrate, the reaction was stopped using 0.5 M H2SO4, and the color reaction was measured at 450 nm.
Immunoelectron microscopy.
L3 were fixed for 30 min in 0.25% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, containing 1% sucrose and were then processed for immunoelectron microscopy as previously described (23). Thin sections of embedded larvae were incubated with polyclonal anti-WSP1 antibodies (1:100) or with serum (1:5) from wild-type and mutant immunized mice, followed by incubation with a suspension of 15-nm gold particles coated with goat anti-rabbit IgG (H + L) or with 10-nm gold particles coated with anti-mouse IgG (Amersham Bioscience, Inc.). Anti-WSP-1 antibody was kindly provided by Mark J. Taylor at the Liverpool School of Tropical Medicine.
Statistical analysis.
Experiments consisted of six mice per group unless otherwise noted. All experiments were performed at least twice, each with equivalent conclusions after statistical analyses; data shown are from one representative experiment. Statistical analysis of the data was performed using the MGLH multifactorial analysis of variance with Systat version 5.2 software (Systat, Evanston, IL) following confirmation that group variances were equivalent. Probability values of less than 0.05 were considered significant.
RESULTS
Protective immunity against O. volvulus does not develop in TLR4-mutant mice.
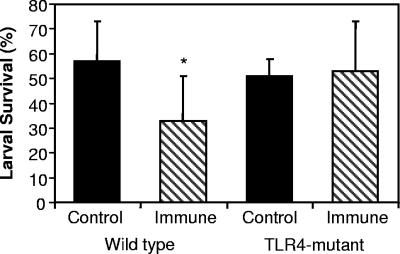
Wild-type C3H/HeOuJ and TLR4-mutant C3H/HeJ mice were immunized with irradiated O. volvulus L3 and then challenged with L3 contained within diffusion chambers to determine if larval survival in immunized mice was affected by the absence of a functional TLR4 receptor. Statistically significant levels of protective immunity developed in immunized wild-type mice, whereas there was no reduction in parasite survival in immunized TLR4-mutant mice, compared to naïve controls (Fig. 1). Therefore, TLR4 is required for the development of a protective immune response to larval O. volvulus.
FIG. 1.
Protective immunity to O. volvulus is absent in TLR4-mutant mice. Naïve mice and mice immunized with irradiated L3 were challenged with 25 L3 contained within a diffusion chamber. Chambers were removed after 3 weeks, and larval survival was assessed. The asterisk represents statistical significance (P ≤ 0.05) from control values; n = 6 for all groups.
Th2 immune responses develop against O. volvulus L3 in TLR4-mutant mice.
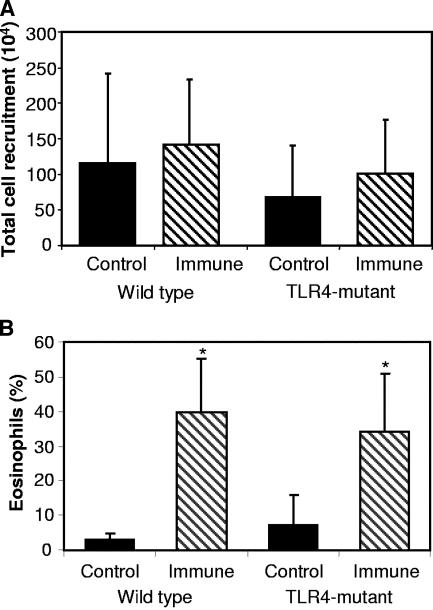
Analyses were performed to determine which components of the protective immune response were compromised in the immunized TLR4-mutant mice. The ability of immunized mice to recruit cells into the diffusion chamber containing the challenge worms was assessed, and no difference in the number of cells recruited into the diffusion chamber between wild-type or TLR4-mutant mice was observed (Fig. 2A). Furthermore, the percentage of eosinophils recruited into the diffusion chambers was equivalent in immunized wild-type and TLR4-mutant mice (Fig. 2B).
FIG. 2.
Total cell and eosinophil recruitment in wild-type and TLR4-mutant mice. The total number of cells recruited into the diffusion chamber was similar between immunized groups (A). Eosinophil recruitment was not different in wild-type and TLR4-mutant mice (B). Asterisks represent statistical differences (P ≤ 0.05) from control values; n = 6 for all groups.
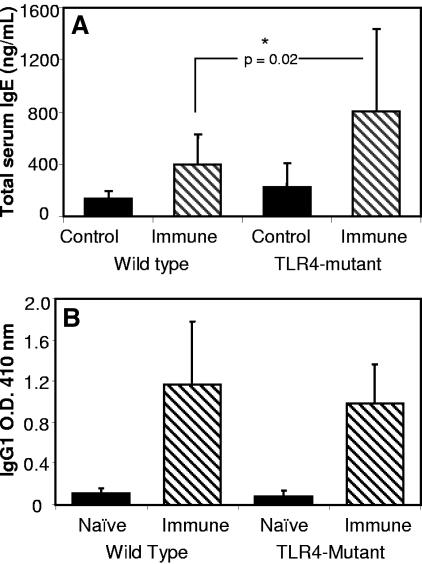
IgE and IgG were measured to determine if the B-cell responses in the TLR4-mutant mice were functional. Total serum IgE levels were significantly higher in immunized TLR4-mutant mice than those levels found in immunized wild-type mice (Fig. 3A). Antigen-specific IgG1 responses were elevated equally in the immunized TLR4-mutant and wild-type mice (Fig. 3B). IgG2a, IgG2b, and IgG3 responses in the immunized TLR4-mutant and wild-type mice were equal to the naïve mouse responses. It was concluded that B-cell responses in the immunized TLR4-mutant mice were unaffected. Furthermore, Th2 responses developed in the TLR4-mutant mice, based on the elevated IgE response and the presence of IgG1 antibodies to parasite antigens.
FIG. 3.
Antibody responses in immunized TLR4-mutant mice. Immunized TLR4-mutant mice have significantly higher total IgE levels than immunized wild-type mice (A) and have IgG1 levels comparable to those of immunized wild-type mice (B). The asterisk represents statistical significance (P ≤ 0.05) from control values; n = 6 for all groups.
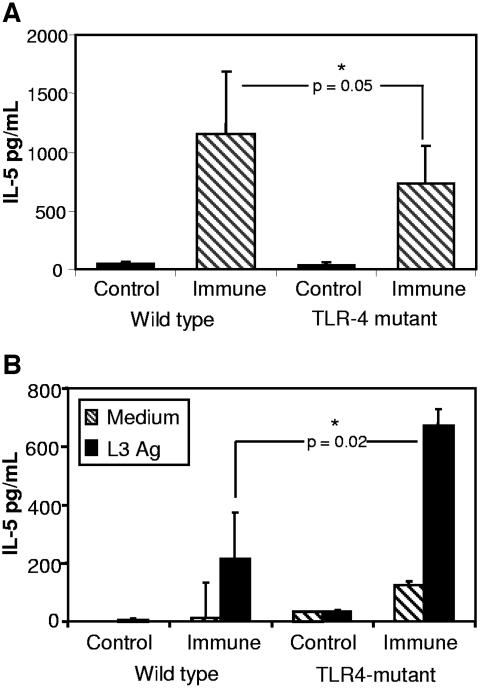
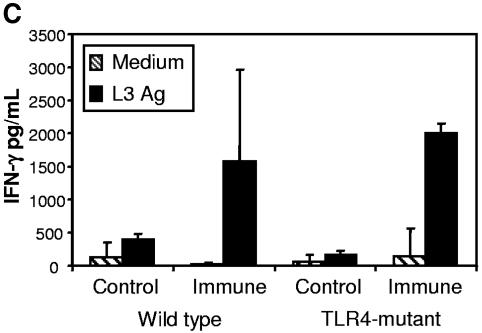
To further evaluate the induction of the protective immune response in the TLR4-mutant mice, cytokines were measured. IL-5 levels were significantly lower in the fluid recovered from the diffusion chambers implanted in immunized TLR4-mutant mice compared to immunized wild-type mice (Fig. 4A). The level of IL-5 found in the immunized TLR4-mutant mice was, however, higher than that found in naïve mice, indicating that the Th2 response in the parasite microenvironment was intact. Cytokine production from spleen cell cultures stimulated with soluble L3 antigens was analyzed, and IL-5 production from immunized TLR4-mutant mice was significantly higher than that found in spleen cells from immunized wild-type mice (Fig. 4B). Additionally, IFN-γ production was the same in immunized wild-type and TLR4-mutant mice (Fig. 4C), indicating that the loss of protective immunity was not due to an increase in the Th1 response.
FIG. 4.
Analysis of cytokine levels in the diffusion chamber and from spleen cell cultures. The level of IL-5 was measured from fluid collected from the diffusion chamber (A). Spleen cells from naïve and immunized mice were cultured in medium alone or with soluble O. volvulus L3 antigen and IL-5 (B), and IFN-γ (C) was measured from cell culture supernatants.
Antibody responses against O. volvulus antigens and Wolbachia in TLR4-mutant mice.
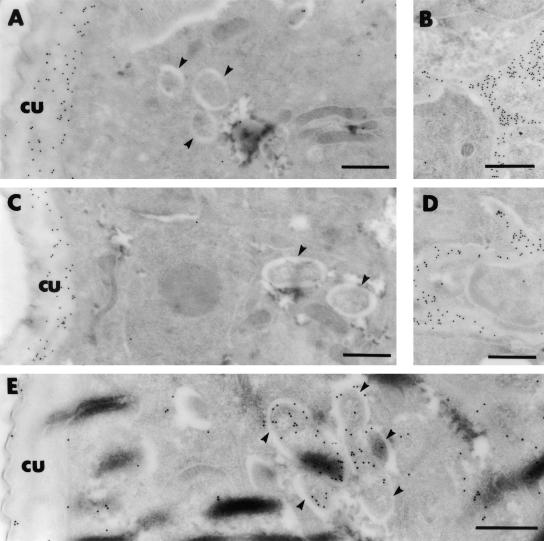
Immunoelectron microscopic analyses were performed to determine if the immune response to Wolbachia, as measured by the localization Wolbachia-specific antibodies, was altered in the absence of TLR4. IgG from both immunized wild-type and TLR4-mutant mice did not bind to Wolbachia (Fig. 5A and C), indicating that even in wild-type mice an IgG response did not develop against Wolbachia. In comparison, control antibodies specifically prepared against WSP-1 (Fig. 5E) bound to the surface of the Wolbachia bacteria within the L3 worms. IgG from immunized wild-type and TLR4-mutant mice bound to the larvae on the median and basal layers of the L3 cuticle (Fig. 5A and C) and the channels connecting the inner spaces of the worm to the cuticle (Fig. 5B and D). The binding of IgE-specific antibodies from both groups was also similar, although it was not clearly confined to particular regions of the larvae (data not shown).
FIG. 5.
Ultrastructural localization of L3 parasite proteins recognized by antibodies from wild-type mice (A and B) or TLR-4 mutant mice (C and D) immunized with radiation-attenuated L3 and challenged with live L3 as determined by immunogold labeling. Sera from wild-type and TLR4-mutant mice have a similar pattern of recognition of the larvae, including the cuticle (cu) and the channels connecting the pseudocoelum to the cuticles. Note that the Wolbachia (arrowhead) is not labeled by either immune serum sample. In comparison, the Wolbachia (arrowhead) is labeled specifically with anti-WSP antibodies (E). Bar, 0.5 nm.
DISCUSSION
The goal of the present study was to determine if TLR4 played a role in the development of protective immunity against larval O. volvulus in mice. This study has demonstrated that C3H/HeJ mice, which have a mutation rendering signaling through TLR4 defective, do not develop immune-mediated larval killing. This finding supports other reports suggesting that TLR4 may be important in the immune response to filarial nematodes. Immunity induced with freeze-thawed microfilariae against larval Brugia malayi was absent in C3H/HeJ mice compared to the C3H/HeN wild-type strain (12). Additionally, TLR4 was proposed to be involved with control of worm embryogenesis of Litomosoides sigmodontis, since the fertility of adult females was increased in C3H/HeJ mice (26). Conversely, resistance to the adult gastrointestinal nematode Trichuris muris was enhanced in C3H/HeJ mice (13), suggesting that TLR4 may interfere with the development of a protective immune response to nematodes. Therefore, based on these reports it is clear that the requirement in mice for TLR4 for the control of nematode infections varies based on the species and potentially the stage of the parasite.
Wolbachia and TLR4 have been implicated in the pathology caused by O. volvulus microfilariae in the eye, such that injection of soluble O. volvulus extracts into the corneal stroma of C3H/HeJ mice induces a significantly lower inflammatory response compared to that in wild-type mice (30). In the current study, it was initially hypothesized that the absence of an immune response against Wolbachia in C3H/HeJ mice may increase parasite survival, since elimination of Wolbachia from filarial worms via drug treatment can lead to a decrease in worm survival (7). It was shown in the present study, however, that L3 survived at equal rates in naïve animals regardless of the presence of a functioning TLR4 receptor. Furthermore, Wolbachia is not the target of the antibody response in immunized wild-type or TLR4-mutant mice, suggesting that the immune response does not target Wolbachia in order to mediate larval killing. Even though the antibody response against Wolbachia proteins may be an effective way to determine disease outcome for lymphatic filariasis (28, 29), it appears to be a poor indicator of protective immunity against larval O. volvulus in mice.
The protective immune response to O. volvulus in mice is Th2 dependent (22). TLR4 has been shown to be required for the induction of a Th2 immune response in mouse allergy models (9, 10) and for the induction of a DC2 phenotype by a glycan from Schistosoma mansoni (33). Wolbachia endosymbionts from O. volvulus and B. malayi contain molecules with LPS-like activity (6, 32), and the inflammatory response to the B. malayi soluble extracts is lost in C3H/HeJ mice (6). Based on this information, we predicted that the protective immune response against O. volvulus could be either (i) decreased in C3H/HeJ if TLR4 were required for the development of the Th2 response or (ii) increased, since there would be an absence of the Th1 response typically associated with Wolbachia and TLR4 signaling. In the current study, the Th2 immune response in immunized animals was significantly increased in C3H/HeJ mice compared to that in wild-type mice; therefore, TLR4 is not required for the development of the Th2 response against O. volvulus. IL-5 production from spleen cell cultures was statistically higher in immunized C3H/HeJ mice. Additionally, levels of eosinophil recruitment, which is required for larval killing (2) and is under Th2 regulation (20), were similar in immunized mice of both strains. Furthermore, there was no change in the Th1 response as shown with IFN-γ production from spleen cell cultures. However, even with a heightened Th2 immune response, protective immunity against the L3 stage was still lost in the TLR4-mutant mice. This is in distinction from reports of chronic infections of C3H/HeJ mice with T. muris, where significantly higher Th2 responses were seen in the TLR4-mutant mice that correlated with increased resistance to the infection (13). The difference in the requirement of TLR4 may be explained by the different anatomical locations in which the worms interact with the immune response or the duration of infections in the mice; T. muris is a chronic gastrointestinal nematode infection, whereas O. volvulus resides subcutaneously short term in a diffusion chamber.
Since B cells express TLR4 (25), it seemed possible that the defect in larval killing occurs due to a change in antibody production. Total serum IgE levels were significantly elevated in immunized C3H/HeJ mice. Furthermore, IgG responses against parasite antigens in both immunized TLR4-mutant and wild-type mice were dominated by IgG1. The elevated IgE response and the antigen-specific IgG1 response thus support the conclusion that the Th2 response in immunized TLR4-mutant mice was intact.
IgG from immunized wild-type and TLR4-mutant mice had the same specificities, based on binding to similar regions in the larvae, as shown by electron microscopy. Neither wild-type nor TLR4-mutant mice mounted an antibody response to Wolbachia. Although with B. malayi and L. sigmodontis it has been reported that the majority of the immune response elicited against Wolbachia is due to the filarial L3 stage (21), this does not appear to be true for O. volvulus. Mice in the present study were immunized with irradiated L3, which may have affected how the immune response developed against Wolbachia or, alternatively, there may be differences in the quantity of Wolbachia organisms between different filarial worms, resulting in different levels of immune recognition.
Two factors, IgE and eosinophils, have been identified to be required for larval killing in mice immunized against O. volvulus (2). Both IgE and eosinophils were present in the immunized TLR4-mutant C3H/HeJ mice at apparently adequate levels for larval killing, which would suggest that other unidentified factors required for larval killing were missing or diminished in these mice. Another explanation for the absence of protective immunity against O. volvulus in immunized TLR4-mutant C3H/HeJ mice may be a failure of activation of the eosinophils recruited into the parasite microenvironment. Eosinophils constitutively express TLR4 (24), and although eosinophils were recruited into the environment of the worms, without TLR4 it is possible that the eosinophils were not activated to kill the larvae. Alternatively, another cell population expressing TLR4, such as neutrophils or macrophages, may be required for activation of eosinophils; therefore, the requirement of TLR4 for activation of eosinophils may be indirect.
In conclusion, a clear requirement for TLR4 in immunity to larval O. volvulus L3 in mice was demonstrated. However, unlike other reports, TLR4 is not required for the development of the Th2 response. Furthermore, Wolbachia does not appear to be the target of the protective immune response, nor does the endosymbiont appear to affect the Th1/Th2 balance of the immune response. The specific requirement of TLR4 is not antibody production or cell recruitment, thus leaving the specific function of TLR4 in the protective immune response against O. volvulus unknown.
Acknowledgments
We thank Shalom Leon, Ann Marie Galioto, Jessica Ligas, De'Broski Herbert, Chun-chi Wang, Yelena Oksov, and Jing Liu for their technical support.
This work was supported in part by NIH grants AI042328 and AI47189.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Abraham, D., A. M. Lange, W. Yutanawiboonchai, M. Trpis, J. W. Dickerson, B. Swenson, and M. L. Eberhard. 1993. Survival and development of larval Onchocerca volvulus in diffusion chambers implanted in primate and rodent hosts. J. Parasitol. 79:571-582. [PubMed] [Google Scholar]
- 2.Abraham, D., O. Leon, S. Schnyder-Candrian, C. C. Wang, A. M. Galioto, L. A. Kerepesi, J. J. Lee, and S. Lustigman. 2004. Immunoglobulin E and eosinophil-dependent protective immunity to larval Onchocerca volvulus in mice immunized with irradiated larvae. Infect. Immun. 72:810-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira, S. 2003. Mammalian Toll-like receptors. Curr. Opin. Immunol. 15:5-11. [DOI] [PubMed] [Google Scholar]
- 4.Brattig, N. W. 2004. Pathogenesis and host responses in human onchocerciasis: impact of Onchocerca filariae and Wolbachia endobacteria. Microbes Infect. 6:113-128. [DOI] [PubMed] [Google Scholar]
- 5.Brattig, N. W., C. Bazzocchi, C. J. Kirschning, N. Reiling, D. W. Buttner, F. Ceciliani, F. Geisinger, H. Hochrein, M. Ernst, H. Wagner, C. Bandi, and A. Hoerauf. 2004. The major surface protein of Wolbachia endosymbionts in filarial nematodes elicits immune responses through TLR2 and TLR4. J. Immunol. 173:437-445. [DOI] [PubMed] [Google Scholar]
- 6.Brattig, N. W., U. Rathjens, M. Ernst, F. Geisinger, A. Renz, and F. W. Tischendorf. 2000. Lipopolysaccharide-like molecules derived from Wolbachia endobacteria of the filaria Onchocerca volvulus are candidate mediators in the sequence of inflammatory and antiinflammatory responses of human monocytes. Microbes Infect. 2:1147-1157. [DOI] [PubMed] [Google Scholar]
- 7.Chirgwin, S. R., J. M. Nowling, S. U. Coleman, and T. R. Klei. 2003. Brugia pahangi and Wolbachia: the kinetics of bacteria elimination, worm viability, and host responses following tetracycline treatment. Exp. Parasitol. 103:16-26. [DOI] [PubMed] [Google Scholar]
- 8.Cooper, P. J., T. Mancero, M. Espinel, C. Sandoval, R. Lovato, R. H. Guderian, and T. B. Nutman. 2001. Early human infection with Onchocerca volvulus is associated with an enhanced parasite-specific cellular immune response. J. Infect. Dis. 183:1662-1668. [DOI] [PubMed] [Google Scholar]
- 9.Dabbagh, K., M. E. Dahl, P. Stepick-Biek, and D. B. Lewis. 2002. Toll-like receptor 4 is required for optimal development of Th2 immune responses: role of dendritic cells. J. Immunol. 168:4524-4530. [DOI] [PubMed] [Google Scholar]
- 10.Eisenbarth, S. C., D. A. Piggott, J. W. Huleatt, I. Visintin, C. A. Herrick, and K. Bottomly. 2002. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 196:1645-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer, P., I. Bonow, D. W. Buttner, I. H. Kamal, and E. Liebau. 2003. An aspartate aminotransferase of Wolbachia endobacteria from Onchocerca volvulus is recognized by IgG1 antibodies from residents of endemic areas. Parasitol. Res. 90:38-47. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi, Y., K. Nakagaki, S. Nogami, B. Hammerberg, and H. Tanaka. 1989. Protective immunity against Brugia malayi infective larvae in mice. I. Parameters of active and passive immunity. Am. J. Trop. Med. Hyg. 41:650-656. [DOI] [PubMed] [Google Scholar]
- 13.Helmby, H., and R. K. Grencis. 2003. Essential role for TLR4 and MyD88 in the development of chronic intestinal nematode infection. Eur. J. Immunol. 33:2974-2979. [DOI] [PubMed] [Google Scholar]
- 14.Hise, A. G., I. Gillette-Ferguson, and E. Pearlman. 2003. Immunopathogenesis of Onchocerca volvulus keratitis (river blindness): a novel role for TLR4 and endosymbiotic Wolbachia bacteria. J. Endotoxin Res. 9:390-394. [DOI] [PubMed] [Google Scholar]
- 15.Hoerauf, A., and N. Brattig. 2002. Resistance and susceptibility in human onchocerciasis—beyond Th1 vs. Th2. Trends Parasitol. 18:25-31. [DOI] [PubMed] [Google Scholar]
- 16.Hoerauf, A., D. W. Buttner, O. Adjei, and E. Pearlman. 2003. Onchocerciasis. BMJ 326:207-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoerauf, A., S. Mand, L. Volkmann, M. Buttner, Y. Marfo-Debrekyei, M. Taylor, O. Adjei, and D. W. Buttner. 2003. Doxycycline in the treatment of human onchocerciasis: kinetics of Wolbachia endobacteria reduction and of inhibition of embryogenesis in female Onchocerca worms. Microbes Infect. 5:261-273. [DOI] [PubMed] [Google Scholar]
- 18.Hoerauf, A., L. Volkmann, C. Hamelmann, O. Adjei, I. B. Autenrieth, B. Fleischer, and D. W. Buttner. 2000. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet 355:1242-1243. [DOI] [PubMed] [Google Scholar]
- 19.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 20.Kopf, M., F. Brombacher, P. D. Hodgkin, A. J. Ramsay, E. A. Milbourne, W. J. Dai, K. S. Ovington, C. A. Behm, G. Kohler, I. G. Young, and K. I. Matthaei. 1996. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 4:15-24. [DOI] [PubMed] [Google Scholar]
- 21.Lamb, T. J., L. Le Goff, A. Kurniawan, D. B. Guiliano, K. Fenn, M. L. Blaxter, A. F. Read, and J. E. Allen. 2004. Most of the response elicited against Wolbachia surface protein in filarial nematode infection is due to the infective larval stage. J. Infect. Dis. 189:120-127. [DOI] [PubMed] [Google Scholar]
- 22.Lange, A. M., W. Yutanawiboonchai, P. Scott, and D. Abraham. 1994. IL-4- and IL-5-dependent protective immunity to Onchocerca volvulus infective larvae in BALB/cBYJ mice. J. Immunol. 153:205-211. [PubMed] [Google Scholar]
- 23.Lustigman, S., B. Brotman, T. Huima, and A. M. Prince. 1991. Characterization of an Onchocerca volvulus cDNA clone encoding a genus specific antigen present in infective larvae and adult worms. Mol. Biochem. Parasitol. 45:65-75. [DOI] [PubMed] [Google Scholar]
- 24.Nagase, H., S. Okugawa, Y. Ota, M. Yamaguchi, H. Tomizawa, K. Matsushima, K. Ohta, K. Yamamoto, and K. Hirai. 2003. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J. Immunol. 171:3977-3982. [DOI] [PubMed] [Google Scholar]
- 25.Ogata, H., I. Su, K. Miyake, Y. Nagai, S. Akashi, I. Mecklenbrauker, K. Rajewsky, M. Kimoto, and A. Tarakhovsky. 2000. The toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J. Exp. Med. 192:23-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfarr, K. M., K. Fischer, and A. Hoerauf. 2003. Involvement of Toll-like receptor 4 in the embryogenesis of the rodent filaria Litomosoides sigmodontis. Med. Microbiol. Immunol. (Berlin) 192:53-56. [DOI] [PubMed] [Google Scholar]
- 27.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 28.Punkosdy, G. A., D. G. Addiss, and P. J. Lammie. 2003. Characterization of antibody responses to Wolbachia surface protein in humans with lymphatic filariasis. Infect. Immun. 71:5104-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Punkosdy, G. A., V. A. Dennis, B. L. Lasater, G. Tzertzinis, J. M. Foster, and P. J. Lammie. 2001. Detection of serum IgG antibodies specific for Wolbachia surface protein in rhesus monkeys infected with Brugia malayi. J. Infect. Dis. 184:385-389. [DOI] [PubMed] [Google Scholar]
- 30.Saint Andre, A., N. M. Blackwell, L. R. Hall, A. Hoerauf, N. W. Brattig, L. Volkmann, M. J. Taylor, L. Ford, A. G. Hise, J. H. Lass, E. Diaconu, and E. Pearlman. 2002. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science 295:1892-1895. [DOI] [PubMed] [Google Scholar]
- 31.Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947-950. [DOI] [PubMed] [Google Scholar]
- 32.Taylor, M. J., H. F. Cross, and K. Bilo. 2000. Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J. Exp. Med. 191:1429-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas, P. G., M. R. Carter, O. Atochina, A. A. Da'Dara, D. Piskorska, E. McGuire, and D. A. Harn. 2003. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a toll-like receptor 4-dependent mechanism. J. Immunol. 171:5837-5841. [DOI] [PubMed] [Google Scholar]
- 34.Trpis, M., G. A. Scoles, and R. H. Struble. 1993. Cryopreservation of infective larvae of Onchocerca volvulus (Filarioidea: Onchocercidae). J. Parasitol. 79:695-700. [PubMed] [Google Scholar]
- 35.Turaga, P. S., T. J. Tierney, K. E. Bennett, M. C. McCarthy, S. C. Simonek, P. A. Enyong, D. W. Moukatte, and S. Lustigman. 2000. Immunity to onchocerciasis: cells from putatively immune individuals produce enhanced levels of interleukin-5, gamma interferon, and granulocyte-macrophage colony-stimulating factor in response to Onchocerca volvulus larval and male worm antigens. Infect. Immun. 68:1905-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]