Figure 2.
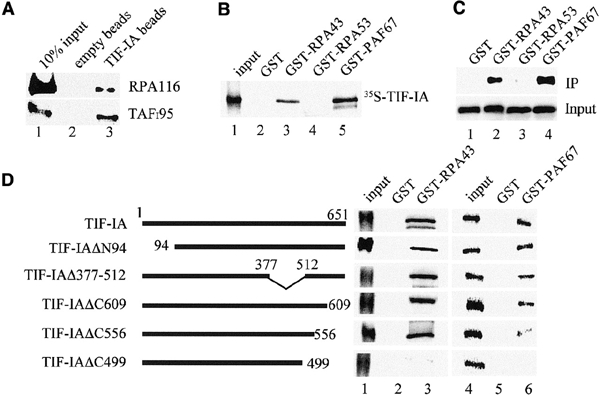
The C-terminus of TIF-IA interacts with RPA43 and PAF67. (A) Interaction of TIF-IA with Pol I and TIF-IB/SL1. Recombinant TIF-IA was incubated with 50 μl of partially purified cellular Pol I or TIF-IB/SL1 and incubated with control beads (lane 2) or bead-bound α-TIF-IA antibodies (lane 3). Associated Pol I and TIF-IB were visualized on western blots using α-RPA116 or α-TAFI95 antibodies. (B) GST pull-down assays. The indicated GST fusion proteins were immobilized on glutathione–Sepharose and incubated with 35S-labeled TIF-IA. TIF-IA bound to GST (lane 2), GST–RPA43 (lane 3), GST–RPA53 (lane 4) and GST–PAF67 (lane 5) was visualized by autoradiography. (C) RPA43 and PAF67 form a stable complex with TIF-IA. Extracts from E. coli expressing TIF-IA and GST (lane 1), TIF-IA and GST–RPA43 (lane 2), TIF-IA and GST–RPA53 (lane 3) or TIF-IA and GST–PAF67 (lane 4) were assayed on western blots for TIF-IA levels (lower panel). After incubation with glutathione–Sepharose, associated TIF-IA was monitored on western blots (upper panel). (D) Mutational analysis of TIF-IA/Pol I interactions. 35S-labeled TIF-IA and the respective mutants were incubated with immobilized GST (lanes 2 and 5), GST–RPA43 (lane 3) and GST–PAF67 (lane 6). TIF-IA was analyzed by SDS–PAGE and autoradiography. Twenty percent of the input proteins are shown in lanes 1 and 4.
