Figure 2.
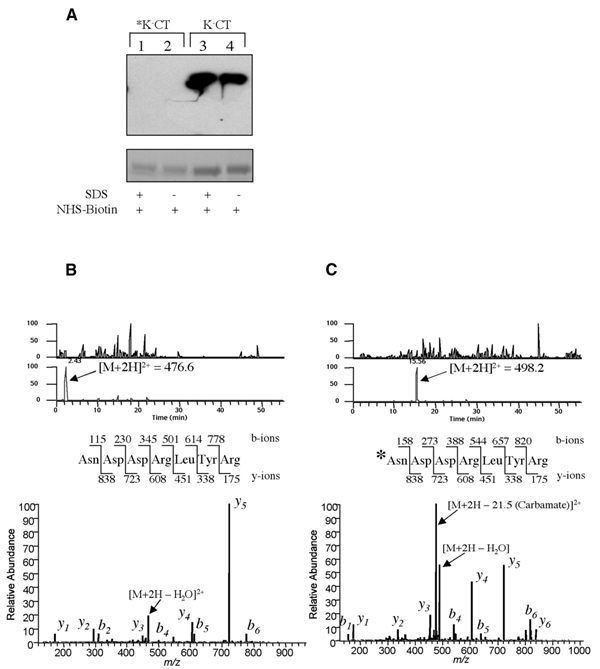
Complete blockade of the N-terminus of the CTA1 chain. (A) Upper: non-reducing SDS–PAGE and avidin-HRP blot of biotin-conjugated K−CT blocked by carbamylation (asterisk, lanes 1 and 2) or unblocked (lanes 3 and 4). Biotinylation was performed in 0.4% SDS (lanes 1 and 3) or in aqueous buffer (lanes 2 and 4). Lower: Coomassie staining of an equivalent gel shows comparable sample loading. (B) The N-terminal peptide of the unblocked K−CT was identified by mass spectroscopy analysis as a free amine. The doubly charged peptide ion (m/z = 476.6) was eluted early in the gradient, and the resulting tandem mass can be matched to the peptide sequence shown without modification. (C) The N-terminus in *K−CT is blocked by carbamylation. The m/z = 476.6 peak in (B) is missing after carbamylation, and a new peak corresponding to the modified doubly charged peptide ion (m/z = 498.2) is detected with an increased retention time. The resulting tandem mass spectrum of this ion shows an N-terminus modified by the mass of a carbamylation (43 Da).
