Figure 1.
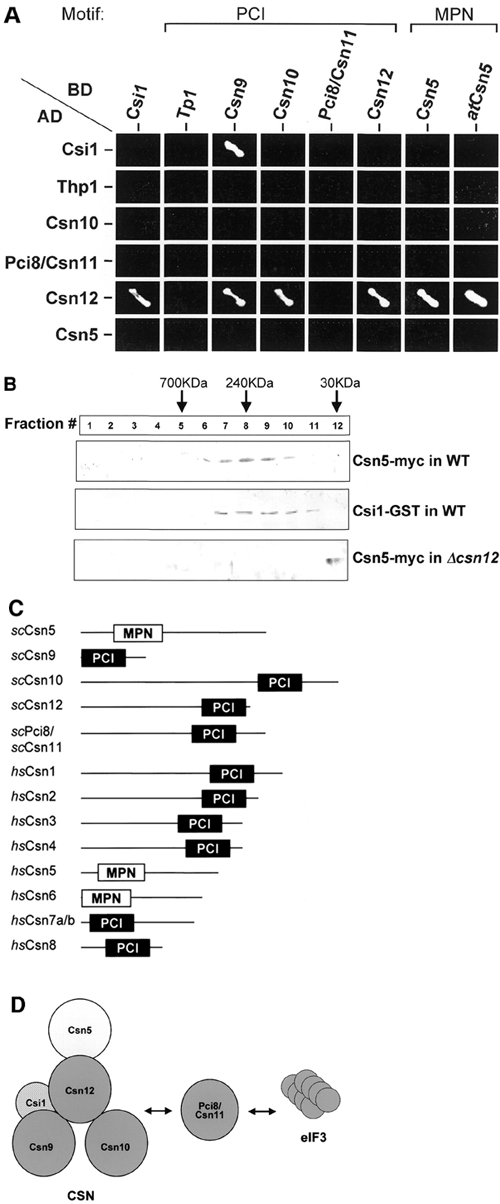
Identification of CSN-like components in S. cerevisiae. (A) Y2H. Pairwise interactions of budding yeast PCI proteins (Csn9, Csn10, Csn12, Pci8/Csn11, Thp1), an MPN protein (Csn5/Rri1) and Csi1. Growth on -ade-his selective media indicates positive interactions. Positive interactions are depicted in (D). Csn5 from plant, atCsn5, is included for comparison. (B) Comigration. Glycerol gradient fractionation of yeast cell extract containing naturally abundant tagged Csn5 or Csi1 in wild-type (WT) or Δcsn12 backgrounds. Csn5 and Csi1 comigrate in a complexed form with an apparent molecular weight of 240 kDa. In Δcsn12, Csn5-myc is detected only in the lowest molecular weight fraction. (C) Structural comparison of identified CSN subunits from budding yeast and human. Cartoons are drawn to scale and depict the signature PCI and MPN domains. See also Supplementary data. (D) Possible model for a CSN-like complex in budding yeast. Csn5, Csn9, Csn10 and Csn12 co-interact with Csn12 playing a pivotal role in the structural organization of this complex (PCI, dark gray; MPN, light gray). Only interactions identified in (A) and (B) are specified. An additional PCI protein, Pci8/Csn11, interacts both with the CSN and eIF3 and may be a shared subunit. A non-PCI/non-MPN protein, Csi1, interacts with Csn9 and Csn12.
