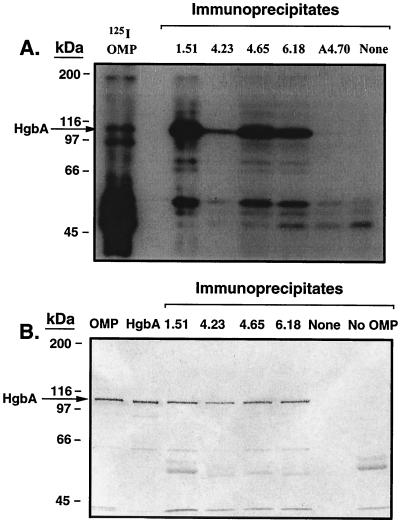
FIG. 1.
Immunoprecipitation of HgbA by anti-HgbA MAbs. (A) Zwittergent-solubilized iodinated OMP was incubated with protein G agarose containing immobilized MAb. Unbound material was removed by washing. The samples were boiled in Laemmli sample buffer and subjected to SDS-PAGE and Coomassie blue staining. The gel was then dried and subjected to autoradiography. Lanes, from left to right: 125I OMP, iodinated OMPs from strain 35000 (100 μg); empty lane; 1.51, 4.23, 4.65, and 6.18, anti-HgbA MAb immunoprecipitates; A4.70, negative control MAb immunoprecipitate to RTX toxin (repeat toxin) of N. meningitidis; None, immunoprecipitation performed in the absence of MAb (negative control). (B). Zwittergent-solubilized unlabeled OMP was subjected to immunoprecipitation as described above and in the text. After SDS-PAGE the gel was subjected to Western blotting with affinity-purified anti-HgbA synthetic peptide IgG. Anti-rabbit alkaline phosphatase was used as the secondary antibody. 5-Bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium was used for detection. Lanes: OMP, strain 35000 OMP (5 μg); purified native HgbA from strain 35000 (200 ng); 1.51, 4.23, 4.65, and 6.18, MAb immunoprecipitates obtained with the OMP antigen; None, immunoprecipitation done in the absence of an MAb; No OMP, MAb 1.51 immunoprecipitation done in the absence of OMP antigen. Immunoprecipitate lanes contained 20 μl (20% of total) per lane.