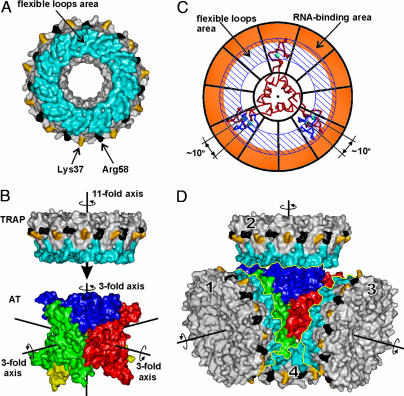
Fig. 5.
AT/TRAP interaction. (A) Molecular surface of TRAP. Surfaces corresponding to tryptophan-binding loops are in blue, and those corresponding to Lys-37 and Arg-58 are in yellow and black, respectively. (B) Molecular surfaces of AT and TRAP are aligned so that one of the four threefold rotational axes of AT 12-mer coincides with the elevenfold rotational axis of TRAP. The AT 12-mer is roughly in the same orientation as in Fig. 1 with its trimers shown in different colors. The three threefold symmetry axes are shown by black lines; the fourth axis (not shown) is roughly perpendicular to the view. (C) Symmetry adjustment. AT trimer is shown as a Cα model (red), and TRAP is shown schematically as a wheel with its RNA-binding area colored orange and the area of flexible trp-binding loops highlighted by diagonal lines. Symmetry adjustment involves rotation of two zinc-binding domains of AT trimer by 10°, to fit the symmetry of the TRAP; these alternate positions are shown in blue. (D) Model for the complex of AT 12-mer with four molecules of TRAP. TRAP and AT are shown as in A and B.