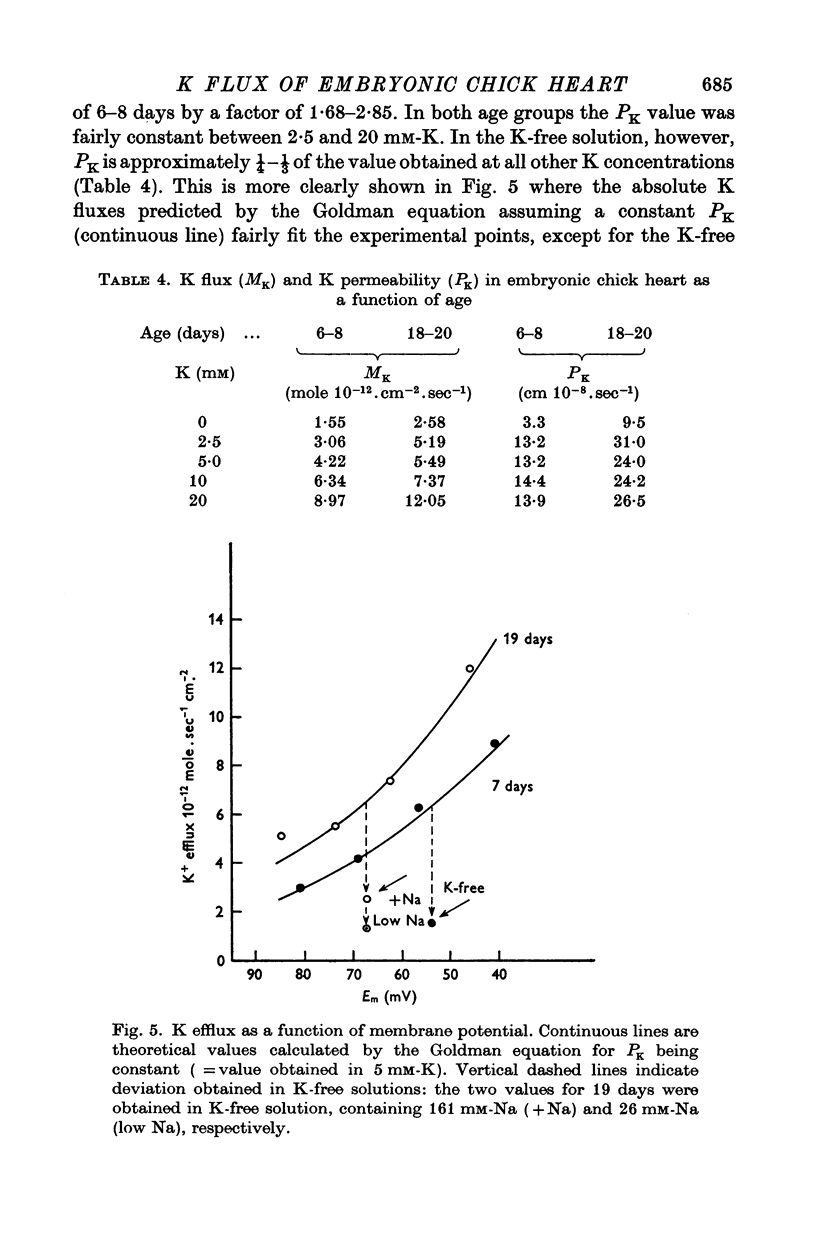
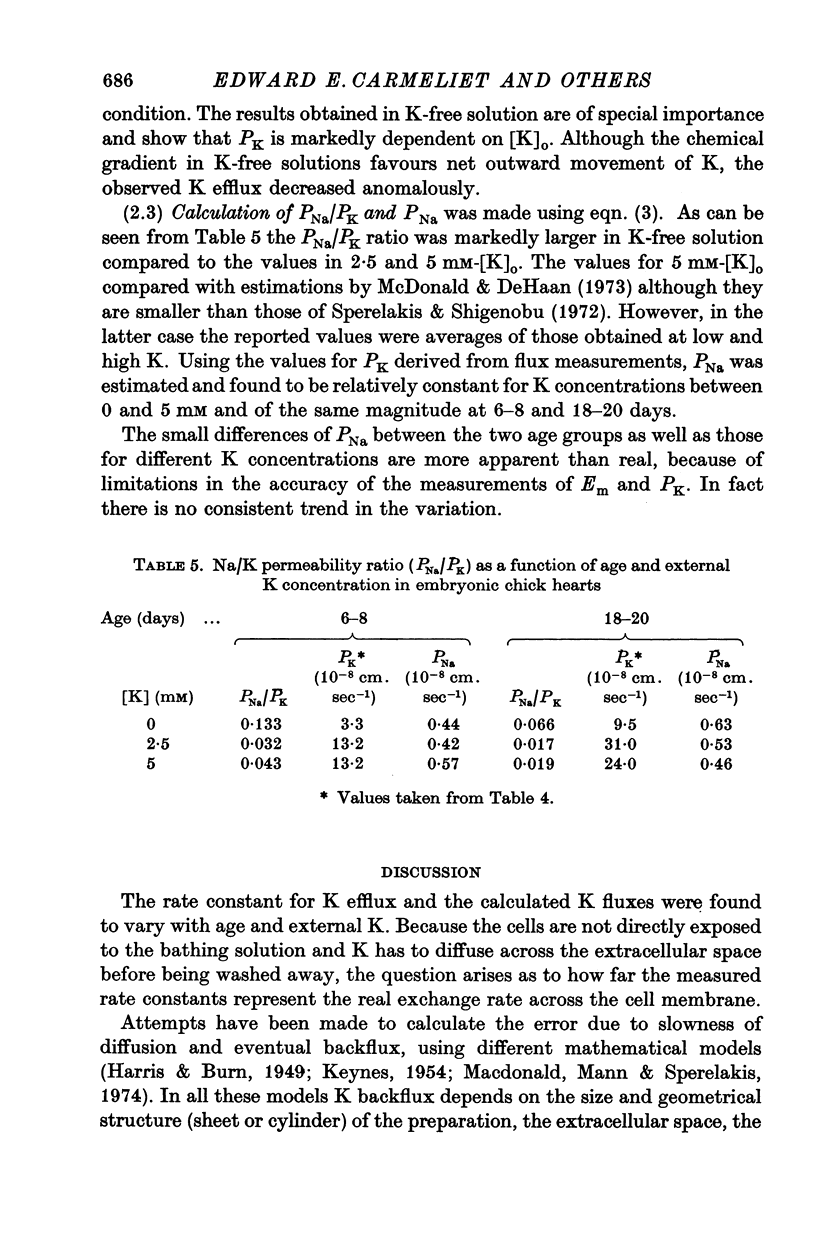
Abstract
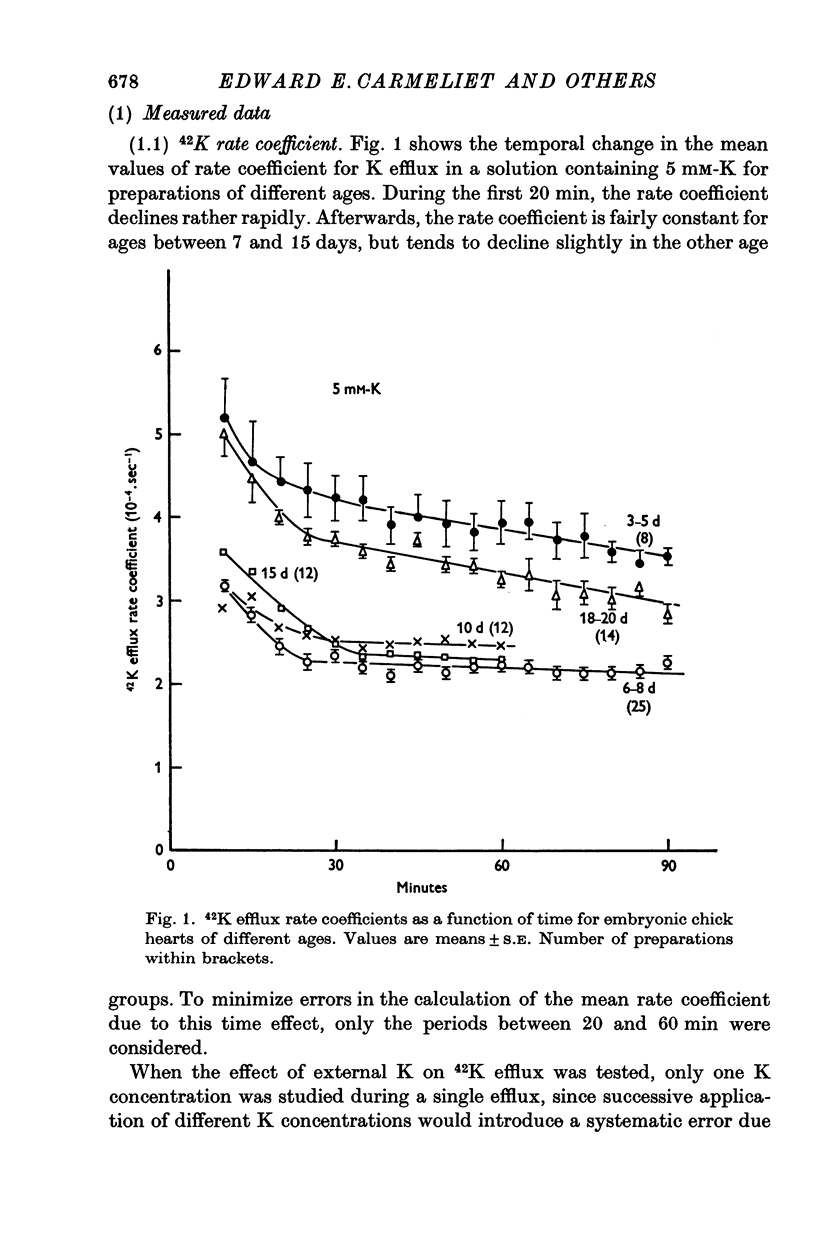
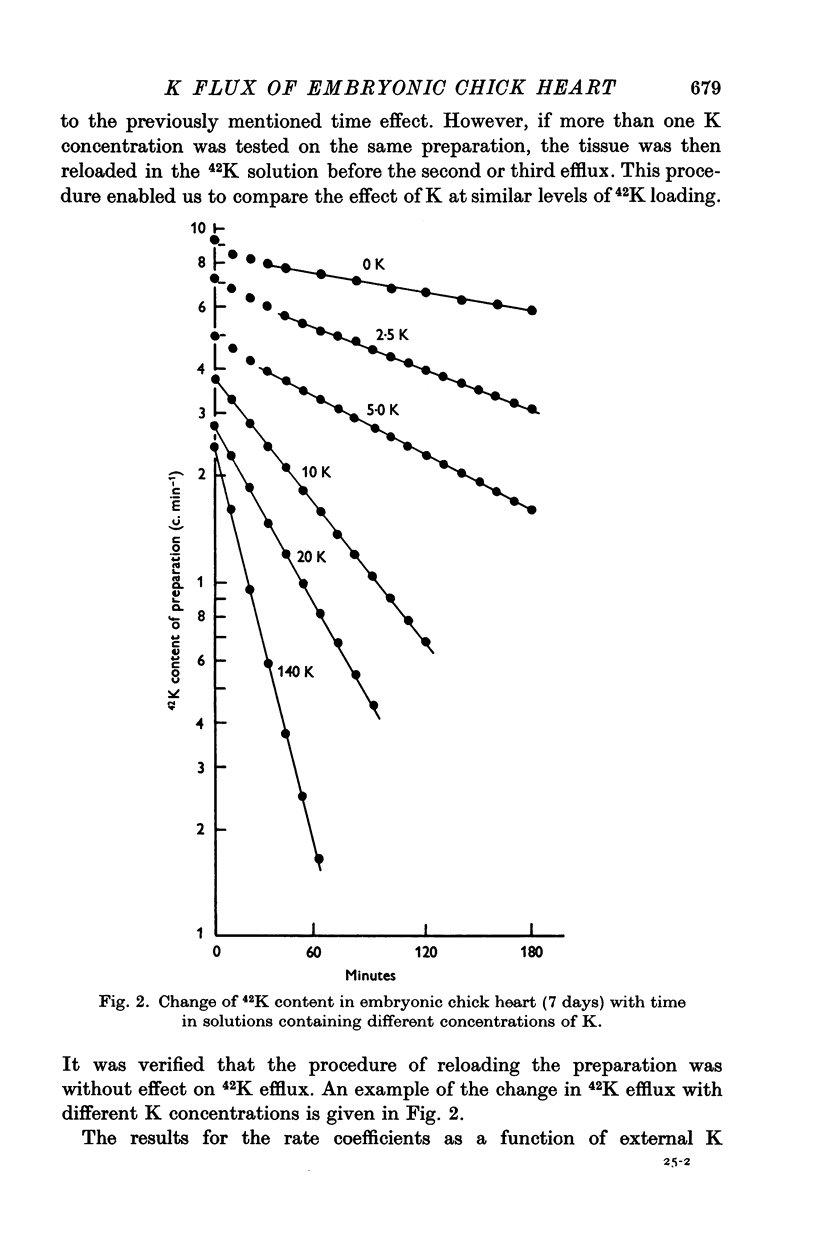
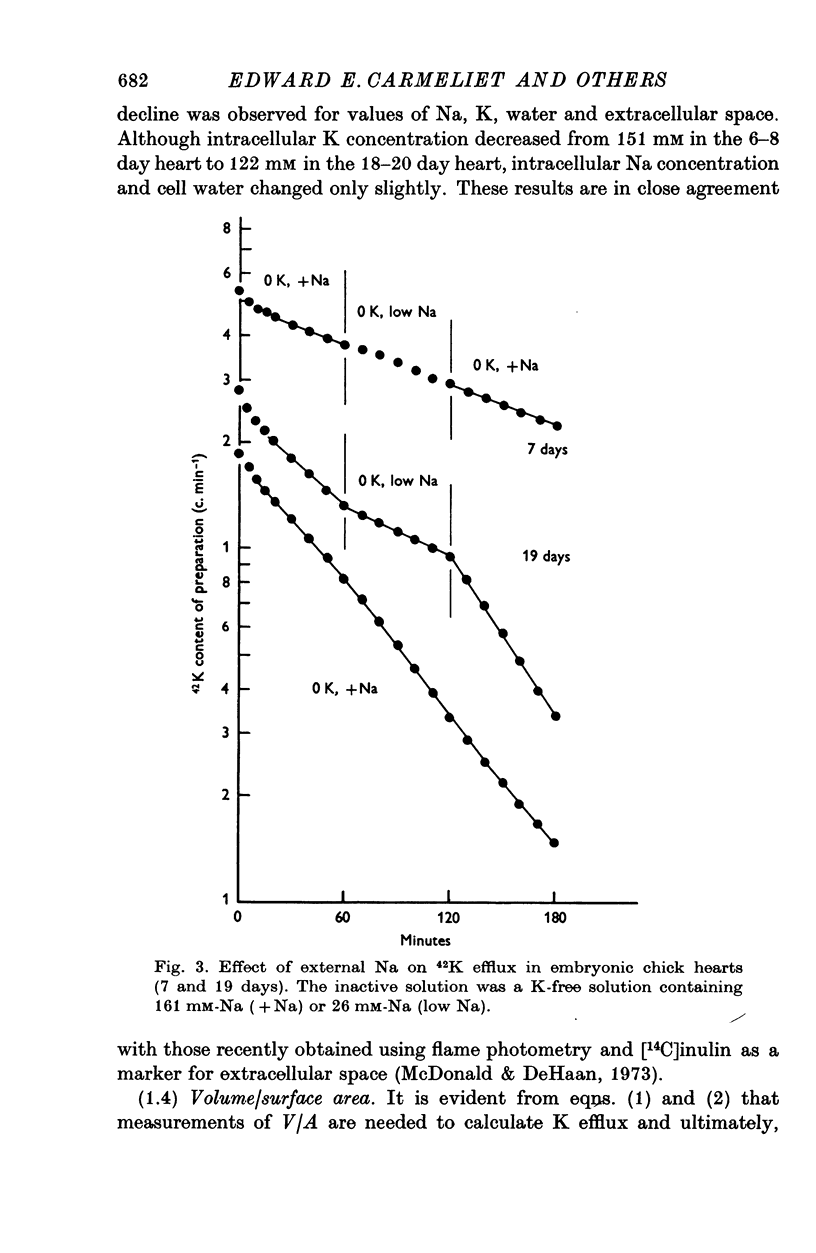
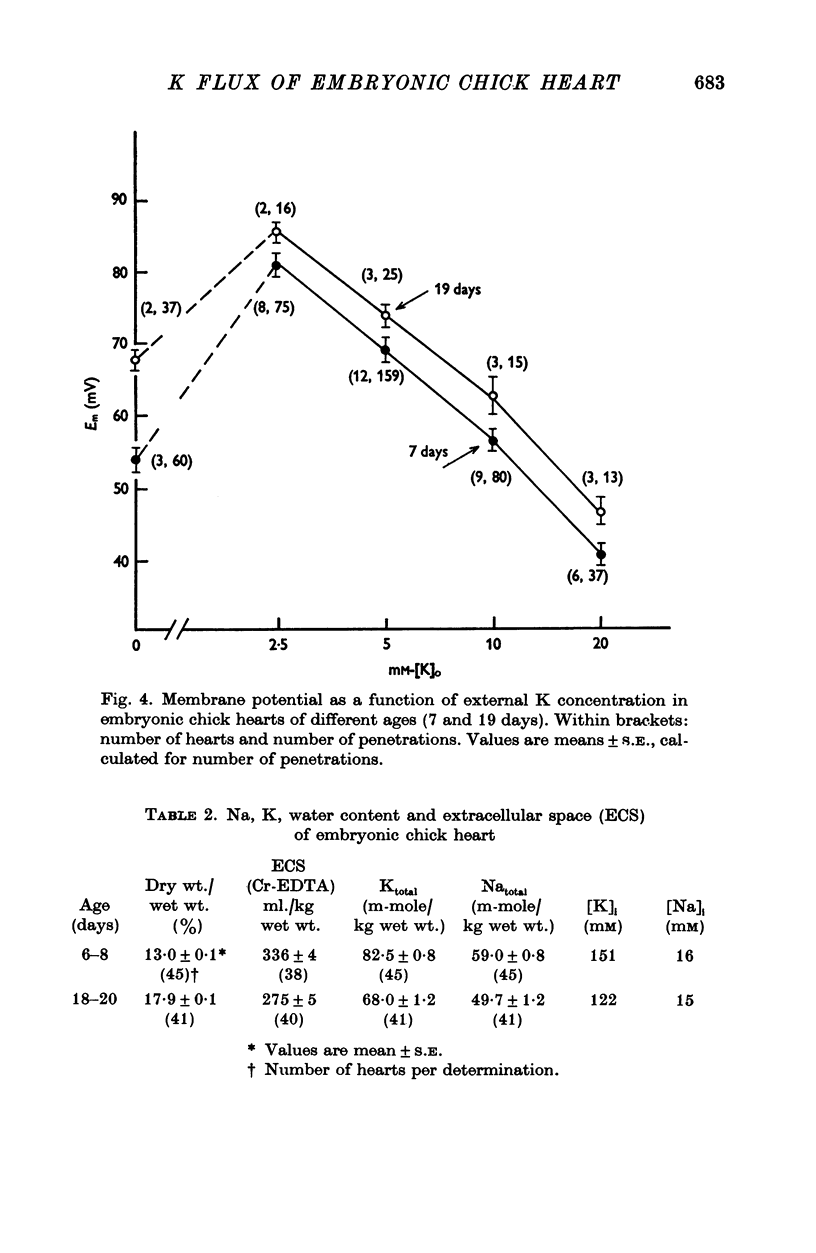
1. The rate coefficient of 42K efflux, the transmembrane potential, the intracellular concentrations of Na and K and the volume/surface area have been measured in embryonic chick hearts of different ages. 2. With respect to age, the rate coefficient for 42K efflux was minimal for preparations from 6-8 day old embryos, and distinctly higher values were obtained for the hearts of 3-5 and 18-20 days. With respect to the effect of external K concentration (Ko), all age groups showed a five- to sevenfold increase in rate coefficient between 2-5 and 140 mM-Ko. The effect of Ko was found to be indepedent of extracellular Na, except in the 18-20 day hearts bathed in K-free solution. 3. Intracellular concentrations of K and Na were found to decrease, membrane potential to increase with age. The volume/surface area measured by stereologic and morphometric techniques did not change with age. 4. The permeability coefficient for K (PK), calculated from the absolute K flux and the measured membrane potentials, was fairly constant for a given age between 2-5 and 20 mM-Ko. In K-free solution, PK was markedly reduced (factor 4). At a given Ko, PK increased twofold between 6-8 and 18-20 days while PNa remained relatively constant.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli T. E., Tieffenberg M., Tosteson D. C. The effect of valinomycin on the ionic permeability of thin lipid membranes. J Gen Physiol. 1967 Dec;50(11):2527–2545. doi: 10.1085/jgp.50.11.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. E., Lieberman M. Increase of potassium flux by valinomycin in embryonic chick heart. Pflugers Arch. 1975 Jul 28;358(3):243–257. doi: 10.1007/BF00587221. [DOI] [PubMed] [Google Scholar]
- Dunand P., Blondel B., Girardier L., Jeanrenaud B. Alpha-aminoisobutyric acid uptake by cultured beating heart cells. Biochim Biophys Acta. 1972 Feb 11;255(2):462–478. doi: 10.1016/0005-2736(72)90150-2. [DOI] [PubMed] [Google Scholar]
- Eisenberg B. R., Kuda A. M., Peter J. B. Stereological analysis of mammalian skeletal muscle. I. Soleus muscle of the adult guinea pig. J Cell Biol. 1974 Mar;60(3):732–754. doi: 10.1083/jcb.60.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G. Activation of the electrogenic sodium pump in guinea-pig auricles by internal sodium ions. J Physiol. 1972 Feb;220(3):565–582. doi: 10.1113/jphysiol.1972.sp009723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L., Lüthi U. Reversal of the potassium entry mechanism in red cells, with and without reversal of the entire pump cycle. J Physiol. 1970 Apr;207(2):371–391. doi: 10.1113/jphysiol.1970.sp009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARSCH M., GREEN J. W. ELECTROLYTE ANALYSES OF CHICK EMBRYONIC FLUIDS AND HEART TISSUES. J Cell Physiol. 1963 Dec;62:319–326. doi: 10.1002/jcp.1030620312. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The resting exchange of radioactive potassium in crab nerve. J Physiol. 1951 Mar;113(1):73–98. doi: 10.1113/jphysiol.1951.sp004557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic fluxes in frog muscle. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):359–382. doi: 10.1098/rspb.1954.0030. [DOI] [PubMed] [Google Scholar]
- KLEIN R. L., EVANS M. L. Effects of ouabain, hypothermia and anoxia on cation fluxes in embryonic chick heart. Am J Physiol. 1961 Apr;200:735–740. doi: 10.1152/ajplegacy.1961.200.4.735. [DOI] [PubMed] [Google Scholar]
- KLEIN R. L. Ontogenesis of K and Na fluxes in embryonic chick heart. Am J Physiol. 1960 Oct;199:613–618. doi: 10.1152/ajplegacy.1960.199.4.613. [DOI] [PubMed] [Google Scholar]
- Lant A. F., Priestland R. N., Whittam R. The coupling of downhill ion movements associated with reversal of the sodium pump in human red cells. J Physiol. 1970 Apr;207(2):291–301. doi: 10.1113/jphysiol.1970.sp009062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Paes de Carvalho A. The Electrophysiological Organization of the Embryonic Chick Heart. J Gen Physiol. 1965 Nov 1;49(2):351–363. doi: 10.1085/jgp.49.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Roggeveen A. E., Purdy J. E., Johnson E. A. Synthetic strands of cardiac muscle: growth and physiological implication. Science. 1972 Feb 25;175(4024):909–911. doi: 10.1126/science.175.4024.909. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Mann J. E., Jr, Sperelakis N. Derivation of general equations describing tracer diffusion in any two-compartment tissue with application to ionic diffusion in cylindrical muscle bundles. J Theor Biol. 1974 May;45(1):107–130. doi: 10.1016/0022-5193(74)90046-0. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., DeHaan R. L. Ion levels and membrane potential in chick heart tissue and cultured cells. J Gen Physiol. 1973 Jan;61(1):89–109. doi: 10.1085/jgp.61.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE E., STORN S. R. CAT HEART MUSCLE IN VITRO. 8. ACTIVE TRANSPORT OF SODIUM IN PAPILLARY MUSCLES. J Gen Physiol. 1965 May;48:957–972. doi: 10.1085/jgp.48.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E., McCallister L. P., Power B. Sterological measurements of cardiac ultrastructures implicated in excitation-contraction coupling. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1465–1466. doi: 10.1073/pnas.68.7.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prignitz R., Müller U., Hoffmeister G. Uber die Bestimmung des Extracellulärraumes mit 51 Cr-EDTA an der Vorhofsmuskulatur des Meerschweinchens. Experientia. 1973 Apr 15;29(4):431–432. doi: 10.1007/BF01926762. [DOI] [PubMed] [Google Scholar]
- SHANES A. M. Electrochemical aspects of physiological and pharmacological action in excitable cells. I. The resting cell and its alteration by extrinsic factors. Pharmacol Rev. 1958 Mar;10(1):59–164. [PubMed] [Google Scholar]
- Sperelakis N. (Na + , K + )-ATPase activity of embryonic chick heart and skeletal muscles as a function of age. Biochim Biophys Acta. 1972 Apr 14;266(1):230–237. doi: 10.1016/0005-2736(72)90137-x. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Shigenobu K. Changes in membrane properties of chick embryonic hearts during development. J Gen Physiol. 1972 Oct;60(4):430–453. doi: 10.1085/jgp.60.4.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira A. W. Cell junctions and their role in transmural diffusion in the embryonic chick heart. Z Zellforsch Mikrosk Anat. 1971;120(4):463–487. doi: 10.1007/BF00340585. [DOI] [PubMed] [Google Scholar]
- Tosteson D. C., Andreoli T. E., Tieffenberg M., Cook P. The effects of macrocyclic compounds on cation transport in sheep red cells and thin and thick lipid membranes. J Gen Physiol. 1968 May;51(5 Suppl):373S+–373S+. [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleugels A., Carmeliet E. Effect of hypoxia on the duration of the action potential in embryonic chick heart. Arch Int Physiol Biochim. 1973 Oct;81(4):775–777. [PubMed] [Google Scholar]
- Weibel E. R. A stereological method for estimating volume and surface of sarcoplasmic reticulum. J Microsc. 1972 Apr;95(2):229–242. doi: 10.1111/j.1365-2818.1972.tb03722.x. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]


