Abstract
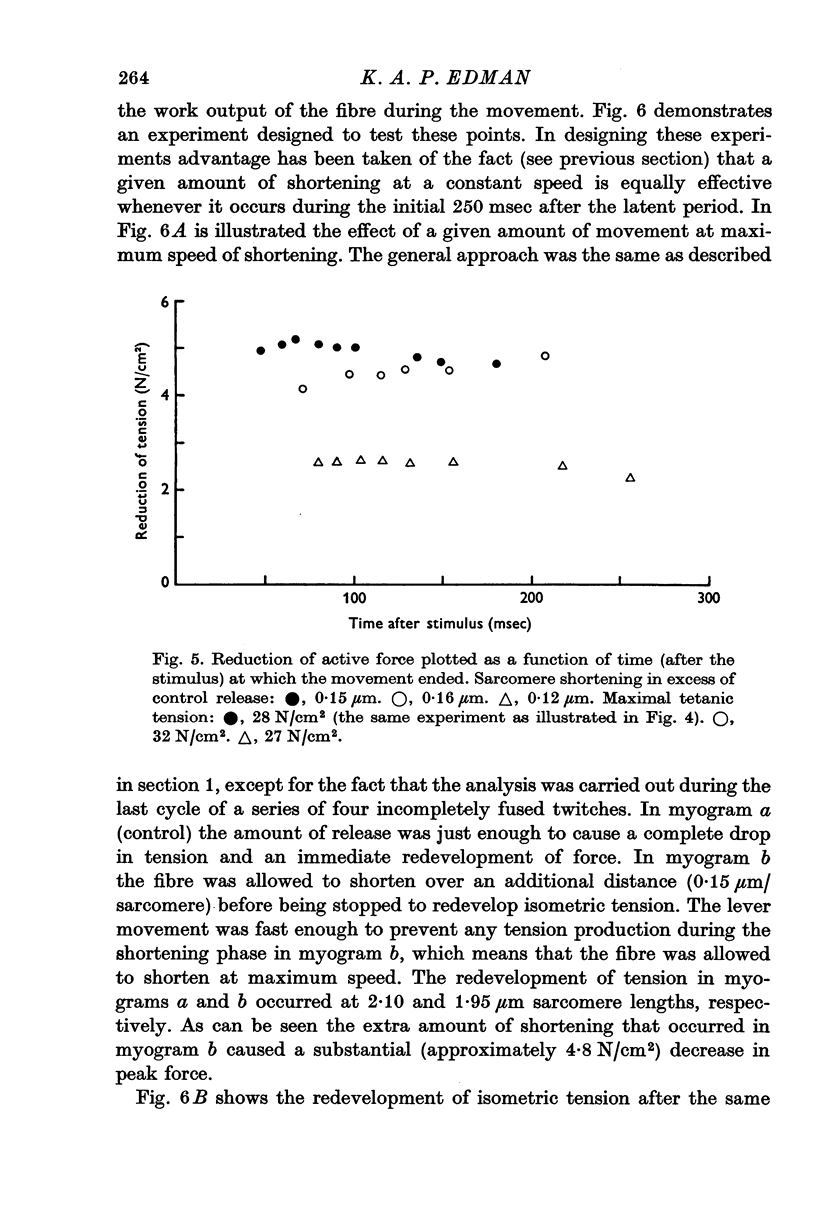
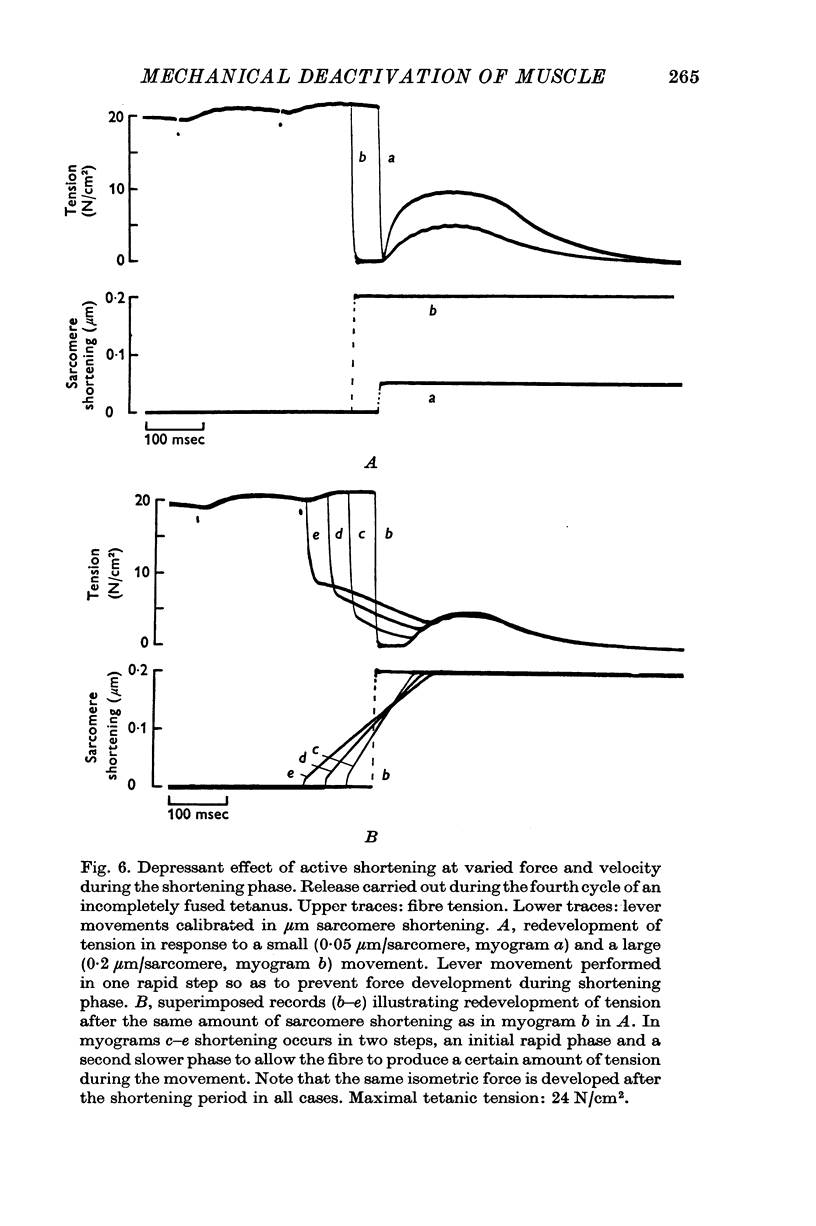
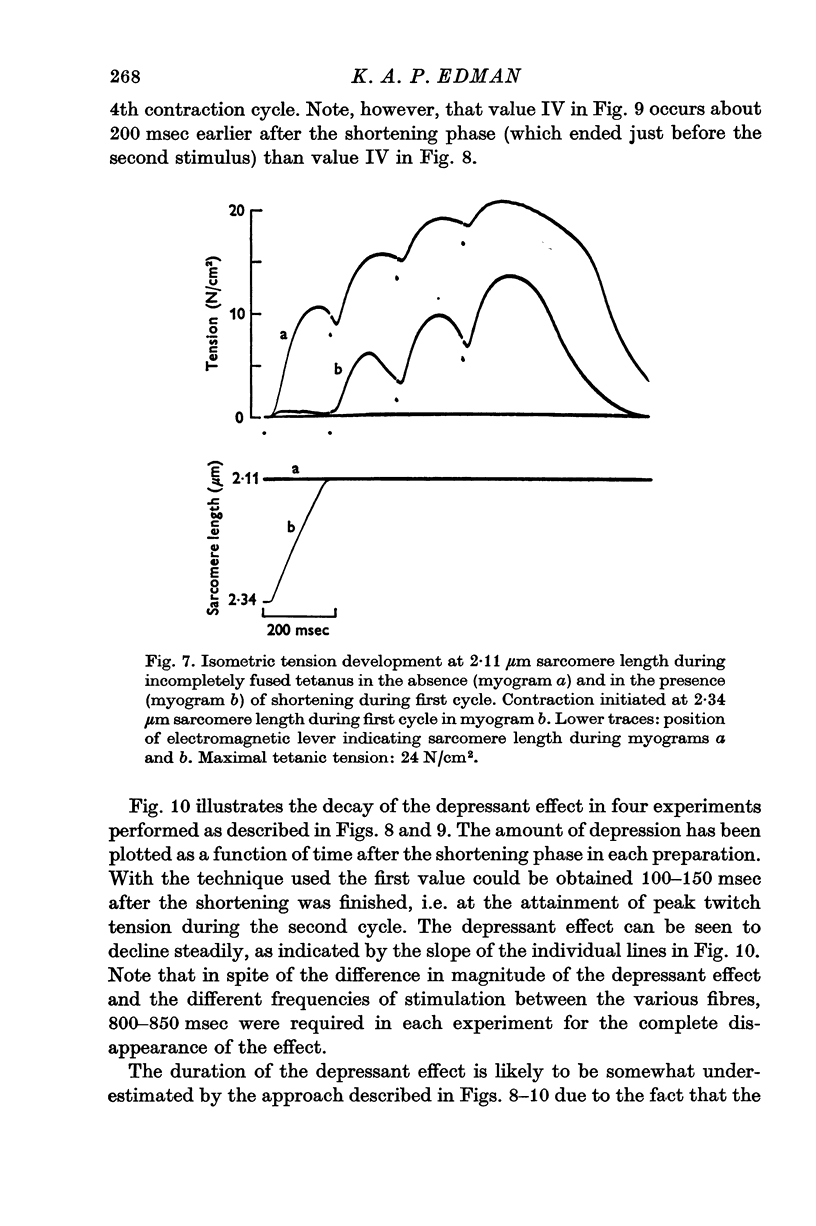
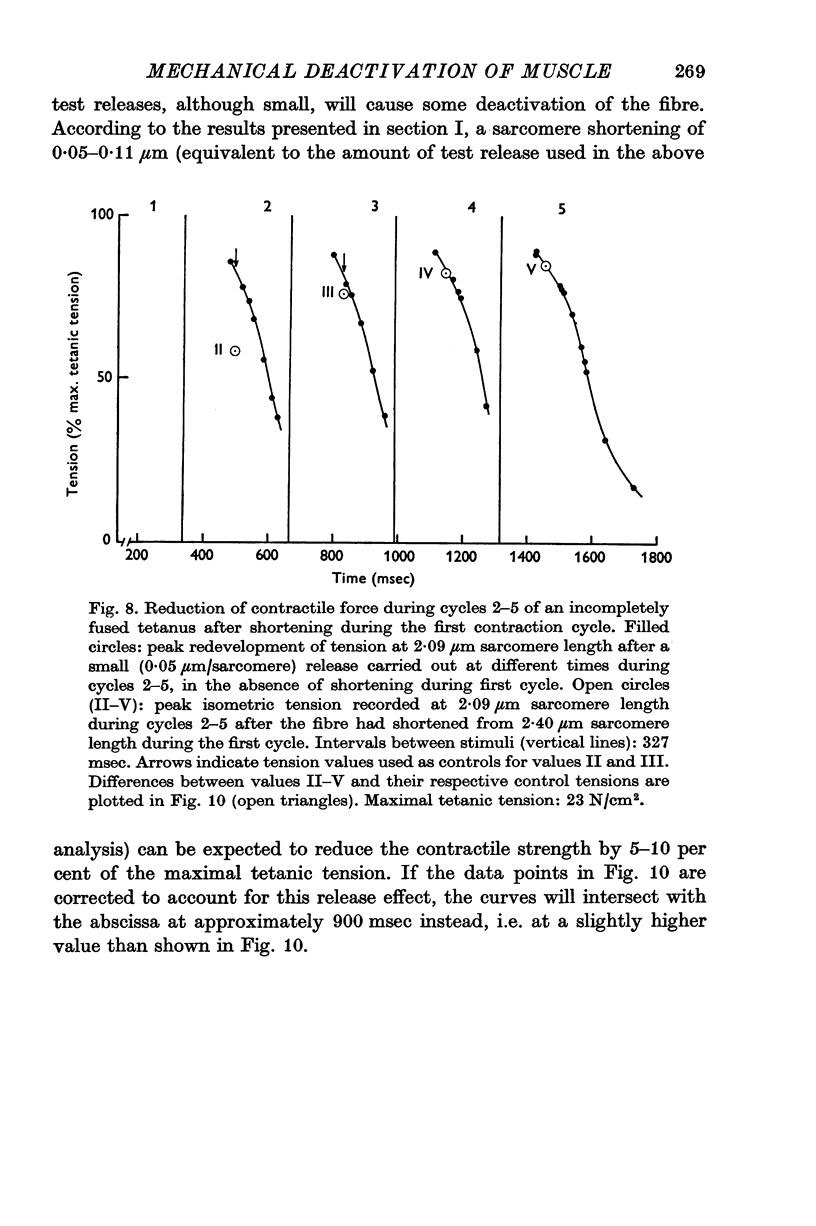
1. The effect of active shortening on the time course and magnitude of isometric tension development during a single twitch and during an incompletely fused tetanus was studied at 0-2-1-2 degres C in isolated semitendinosus muscle fibres of the frog. 2. Active shortening caused a depression of the contractile force without markedly affecting the total duration of the twitch. The depressant effect increased with increasing amounts of sarcomere shortening. Sarcomere shortenings of 0-05 mum and 0-3 mum reduced the twitch force by approximately 5 and 20 percent of the maximal tetanic tension, respectively. 3. A given sarcomere shortening induced the same absolute amount of depression of the contractile strength when the movement was carried out at different times during the initial 200-250 msec after the stimulus. 4. The influence of load and velocity of shortening during the movement phase was studied. Differences in load ranging between zero and 1/3 of the maximal tetanic tension (with concomitant changes in speed of shortening from Vmax to approximately 1/5 of Vmax) did not affect the degree of depression markedly. Underthe conditions studied, the extent of movement appeared to be the only significant determinant of the depressant effect. 5. The reduction in force induced by active shortening persisted for 800-900 msec during an incompletely fused tetanus. 6. It is suggested that the depressant effect is based on a structural change in the myofilament system that is produced as the A and I filaments slide along each other during muscle activity.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonini E., Brunori M. Hemoglobin. Annu Rev Biochem. 1970;39:977–1042. doi: 10.1146/annurev.bi.39.070170.004553. [DOI] [PubMed] [Google Scholar]
- Arlock P. A capacitance indicator for infinitesimal muscular movements. Experientia. 1970 Sep 26;26(9):1046–1047. doi: 10.1007/BF02114187. [DOI] [PubMed] [Google Scholar]
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozler E. Feedback in the contractile mechanism of the frog heart. J Gen Physiol. 1972 Sep;60(3):239–247. doi: 10.1085/jgp.60.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady A. J. Onset of contractility in cardiac muscle. J Physiol. 1966 Jun;184(3):560–580. doi: 10.1113/jphysiol.1966.sp007931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briden K. L., Alpert N. R. The effect of shortening on the time-course of active state decay. J Gen Physiol. 1972 Aug;60(2):202–220. doi: 10.1085/jgp.60.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleworth D. R., Edman K. A. Changes in sarcomere length during isometric tension development in frog skeletal muscle. J Physiol. 1972 Dec;227(1):1–17. doi: 10.1113/jphysiol.1972.sp010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson V. A., Woledge R. C. The thermal effects of shortening in tetanic contractions of frog muscle. J Physiol. 1973 Sep;233(3):659–671. doi: 10.1113/jphysiol.1973.sp010328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Kiessling A. The time course of the active state in relation to sarcomere length and movement studied in single skeletal muscle fibres of the frog. Acta Physiol Scand. 1971 Feb;81(2):182–196. doi: 10.1111/j.1748-1716.1971.tb04891.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Nilsson E. Relationships between force and velocity of shortening in rabbit papillary muscle. Acta Physiol Scand. 1972 Aug;85(4):488–500. doi: 10.1111/j.1748-1716.1971.tb05286.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The relation between sarcomere length and active tension in isolated semitendinosus fibres of the frog. J Physiol. 1966 Mar;183(2):407–417. doi: 10.1113/jphysiol.1966.sp007873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. The mechanical properties of relaxing muscle. J Physiol. 1960 Jun;152:30–47. doi: 10.1113/jphysiol.1960.sp006467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M. Isotonic lengthening and shortening movements of cat soleus muscle. J Physiol. 1969 Oct;204(2):475–491. doi: 10.1113/jphysiol.1969.sp008925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M., Westbury D. R. The mechanical properties of cat soleus muscle during controlled lengthening and shortening movements. J Physiol. 1969 Oct;204(2):461–474. doi: 10.1113/jphysiol.1969.sp008924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöbsis F. F., O'Connor M. J. Calcium release and reabsorption in the sartorius muscle of the toad. Biochem Biophys Res Commun. 1966 Oct 20;25(2):246–252. doi: 10.1016/0006-291x(66)90588-2. [DOI] [PubMed] [Google Scholar]
- Kaufmann R. L., Bayer R. M., Harnasch C. Autoregulation of contractility in the myocardial cell. Displacement as a controlling parameter. Pflugers Arch. 1972;332(2):96–116. [PubMed] [Google Scholar]
- Mommaerts W. F. Energetics of muscular contraction. Physiol Rev. 1969 Jul;49(3):427–508. doi: 10.1152/physrev.1969.49.3.427. [DOI] [PubMed] [Google Scholar]
- PRINGLE J. W. Models of muscle. Symp Soc Exp Biol. 1960;14:41–68. [PubMed] [Google Scholar]
- RITCHIE J. M. The effect of nitrate on the active state of muscle. J Physiol. 1954 Oct 28;126(1):155–168. doi: 10.1113/jphysiol.1954.sp005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., WILKIE D. R. The dynamics of muscular contraction. J Physiol. 1958 Aug 29;143(1):104–113. doi: 10.1113/jphysiol.1958.sp006047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi H. Tension changes during and after stretch in frog muscle fibres. J Physiol. 1972 Aug;225(1):237–253. doi: 10.1113/jphysiol.1972.sp009935. [DOI] [PMC free article] [PubMed] [Google Scholar]


