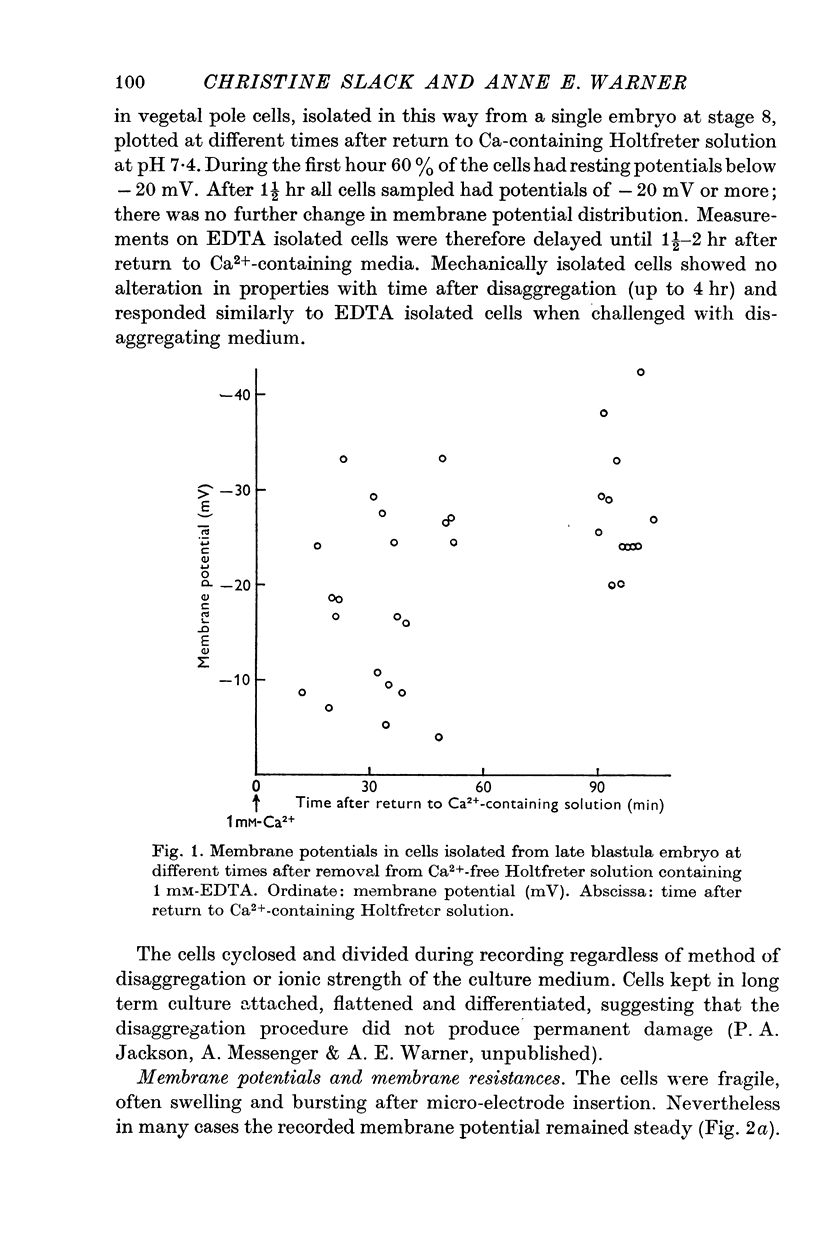
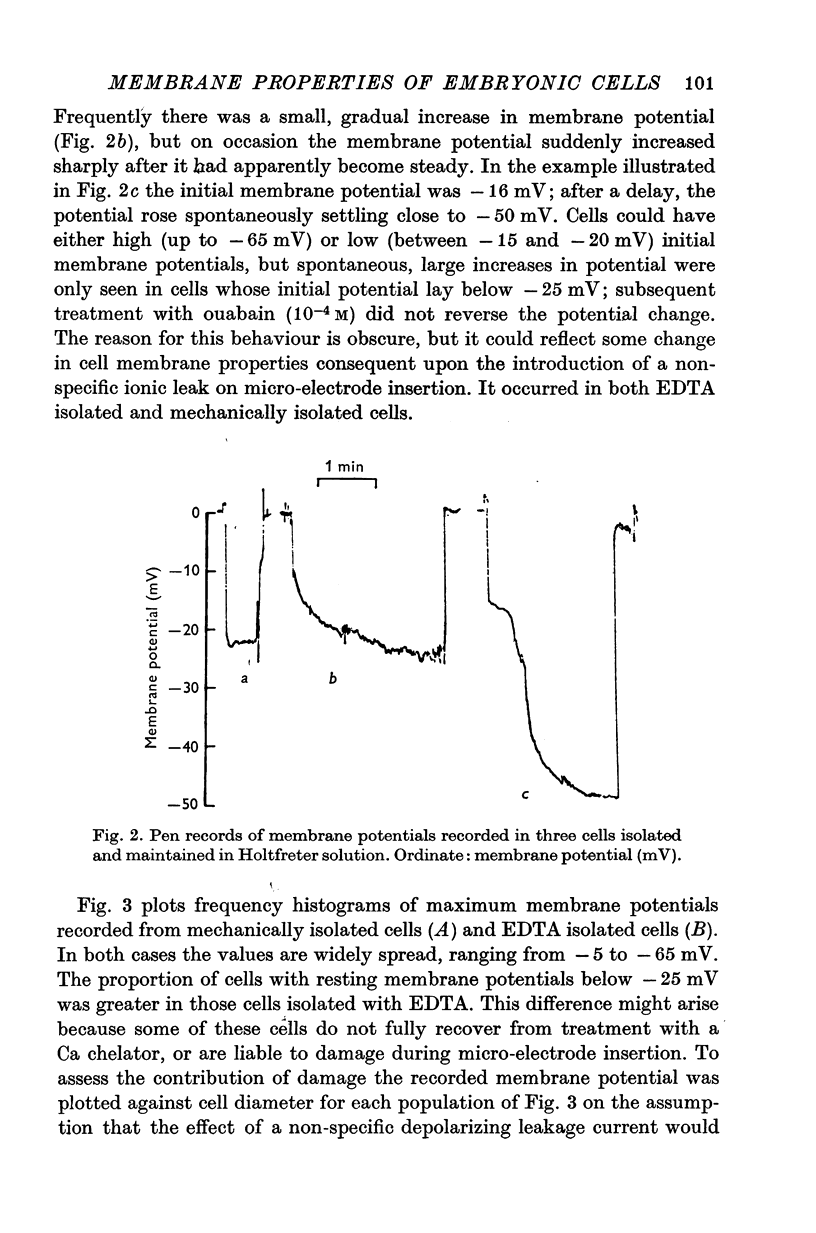
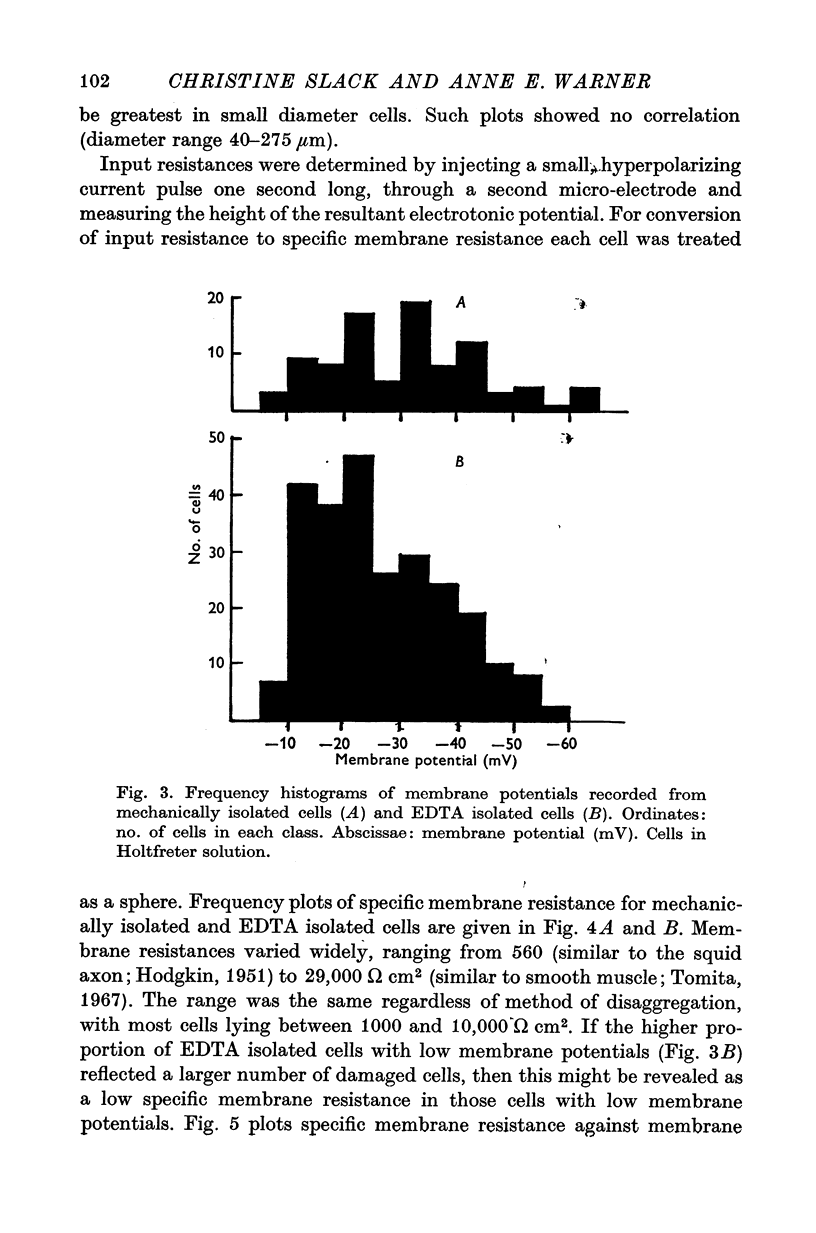
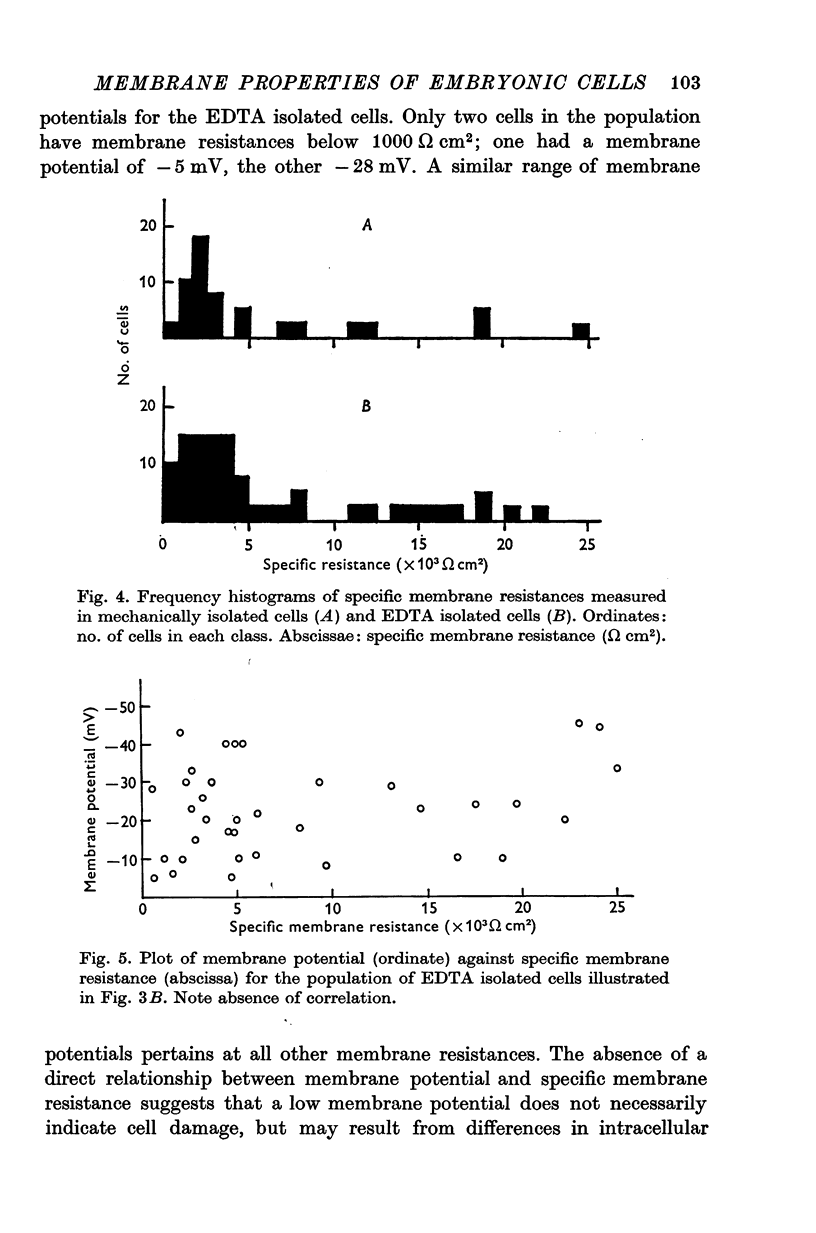
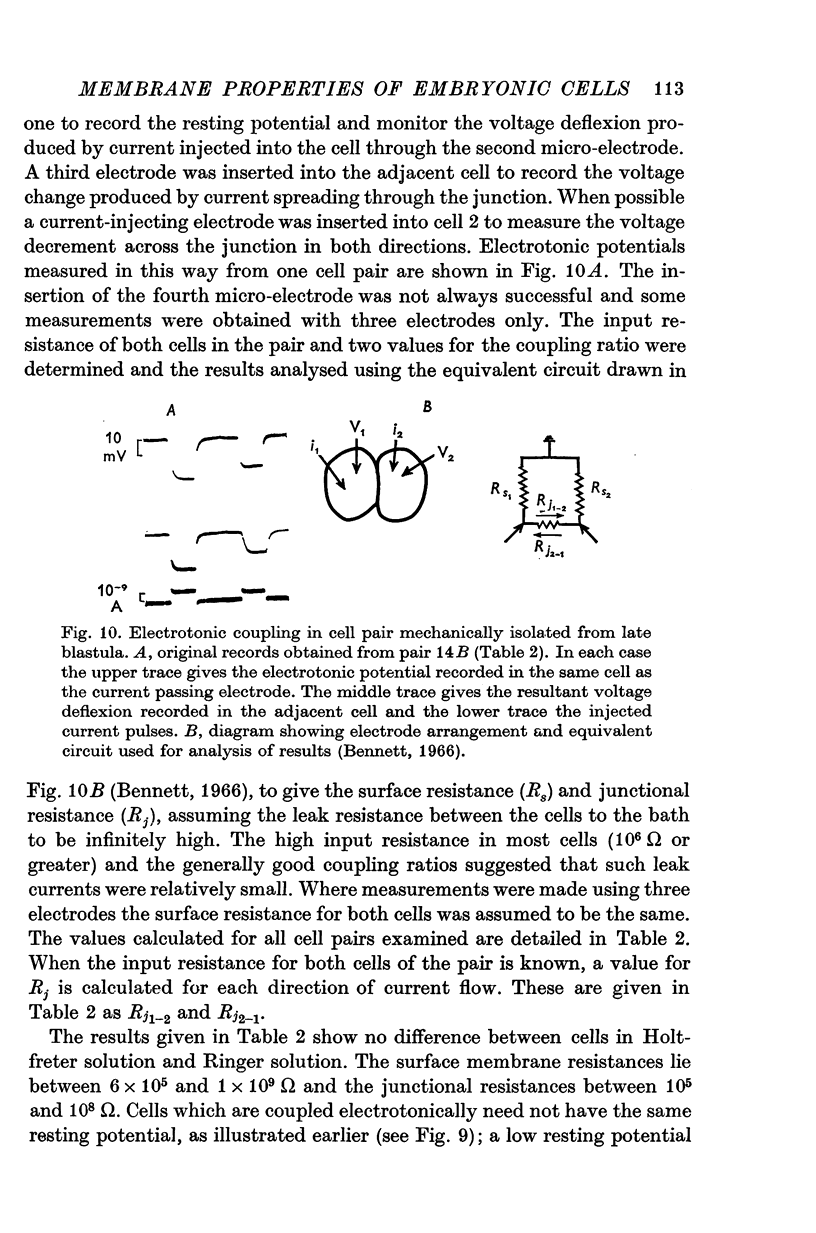
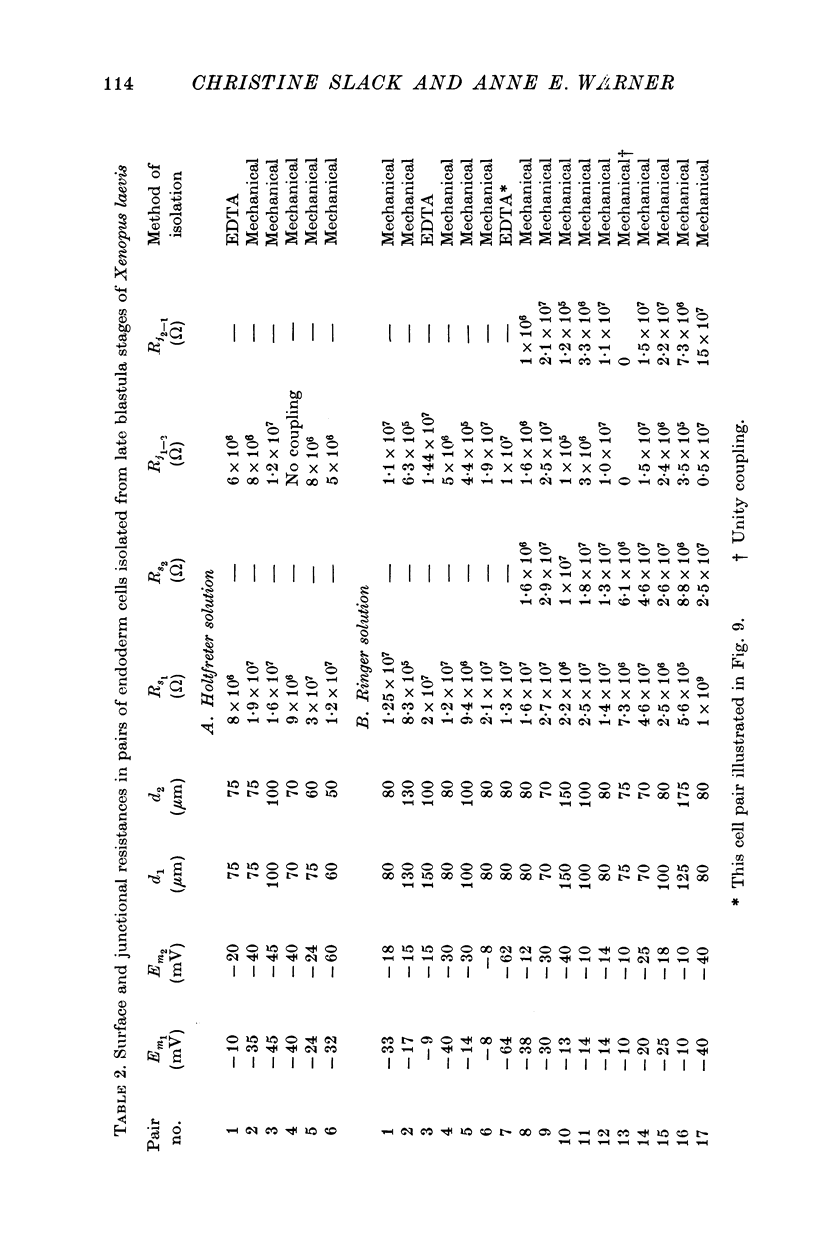
Abstract
1. Some membrane properties of endoderm and mesoderm cells isolated from late blastula stages of Xenopus laevis have been examined using electrophysiological techniques. 2. Cells were isolated by treatment of whole embryos with Ca-free EDTA containing media, or mechanically by micro-dissection, and cultured in Ca-containing Holtfreter solution (60 mM-NaCl) or Ringer solution (120 mM-NaCl). 3. Membrane potentials lay between -6 and -84 mV; specific membrane resistances ranged from 500 to 29,000 omega cm2; there was no difference between EDTA isolated and mechanically isolated cells. 4. Relative and absolute cation and anion conductances varied from cell to cell. Some cells were anion impermeable; the cation conductance ranged from 35 to 300 mumho/cm2. 5. The resting potential of some cells was largely determined by the concentration gradient and membrane permeability of K ions. In other cells the potential was maintained either by some other ion or by an electrogenic pump. [K]i came to approximately 130 mM in Ringer solution (the value pertaining in the intact embryo) and similar to 60 mM in Holtfreter solution. 6. In most pairs and small clumps of cells ionic current spread from one cell to the next; some single cells and groups of cells were uncoupled from their neighbours. 7. The junctional resistance lay between 10(5) and 10(8) omega; it behaved as a linear resistor in most cell pairs studied. In three pairs the intercellular junction showed rectifying properites. 8. By the late blastula stage of development presumptive endoderm and mesoderm cells form a heterogeneous population with widely varying passive membrane properties. The significance of these findings is discussed in relation to current hypotheses for the formation of spatial patterns during differentiation.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTH L. G., BARTH L. J. Differentiation of cells of the Rana pipiens gastrula in unconditioned medium. J Embryol Exp Morphol. 1959 Jun;7:210–222. [PubMed] [Google Scholar]
- Baker P. F., Warner A. E. Intracellular calcium and cell cleavage in early embryos of Xenopus laevis. J Cell Biol. 1972 May;53(2):579–581. doi: 10.1083/jcb.53.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V. Physiology of electrotonic junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):509–539. doi: 10.1111/j.1749-6632.1966.tb50178.x. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Trinkaus J. P. Electrical coupling between embryonic cells by way of extracellular space and specialized junctions. J Cell Biol. 1970 Mar;44(3):592–610. doi: 10.1083/jcb.44.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. Properties of the primary organization field in the embryo of Xenopus laevis. I. Autonomy of cell behaviour at the site of initial organizer formation. J Embryol Exp Morphol. 1972 Aug;28(1):13–26. [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furshpan E. J., Potter D. D. Low-resistance junctions between cells in embryos and tissue culture. Curr Top Dev Biol. 1968;3:95–127. doi: 10.1016/s0070-2153(08)60352-x. [DOI] [PubMed] [Google Scholar]
- Gingell D. Contractile responses at the surface of an amphibian egg. J Embryol Exp Morphol. 1970 Jun;23(3):583–609. [PubMed] [Google Scholar]
- Goodwin B. C., Cohen M. H. A phase-shift model for the spatial and temporal organization of developing systems. J Theor Biol. 1969 Oct;25(1):49–107. doi: 10.1016/s0022-5193(69)80017-2. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The effect of sudden changes in ionic concentrations on the membrane potential of single muscle fibres. J Physiol. 1960 Sep;153:370–385. doi: 10.1113/jphysiol.1960.sp006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. The chloride conductance of frog skeletal muscle. J Physiol. 1960 Apr;151:89–102. [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The pH sensitivity of the chloride conductance of frog skeletal muscle. J Physiol. 1967 Apr;189(3):403–425. doi: 10.1113/jphysiol.1967.sp008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Hori N. Electrical characteristics of Triturus egg cells during cleavage. J Gen Physiol. 1966 May;49(5):1019–1027. doi: 10.1085/jgp.49.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Loewenstein W. R. Ionic communication between early embryonic cells. Dev Biol. 1969 Mar;19(3):228–243. doi: 10.1016/0012-1606(69)90062-1. [DOI] [PubMed] [Google Scholar]
- Kostellow A. B., Morrill G. A. Intracellular sodium ion concentration changes in the early amphibian embryo and the influence on nuclear metabolism. Exp Cell Res. 1968 Jun;50(3):639–644. doi: 10.1016/0014-4827(68)90425-4. [DOI] [PubMed] [Google Scholar]
- Kroeger H., Lezzi M. Regulation of gene action in insect development. Annu Rev Entomol. 1966;11:1–22. doi: 10.1146/annurev.en.11.010166.000245. [DOI] [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. A., Crick F. H., Munro M. A gradient of positional information in an insect, Rhodnius. J Cell Sci. 1972 Nov;11(3):815–853. doi: 10.1242/jcs.11.3.815. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R., Socolar S. J., Higashino S., Kanno Y., Davidson N. Intercellular Communication: Renal, Urinary Bladder, Sensory, and Salivary Gland Cells. Science. 1965 Jul 16;149(3681):295–298. doi: 10.1126/science.149.3681.295. [DOI] [PubMed] [Google Scholar]
- Miyazaki S. I., Takahashi K., Tsuda K., Yoshii M. Analysis of non-linearity observed in the current-voltage relation of the tunicate embryo. J Physiol. 1974 Apr;238(1):55–77. doi: 10.1113/jphysiol.1974.sp010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. F., Slack C. Some bio-electric parameters of early Xenopus embryos. J Embryol Exp Morphol. 1970 Nov;24(3):535–553. [PubMed] [Google Scholar]
- Potter D. D., Furshpan E. J., Lennox E. S. Connections between cells of the developing squid as revealed by electrophysiological methods. Proc Natl Acad Sci U S A. 1966 Feb;55(2):328–336. doi: 10.1073/pnas.55.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman G. G., Perry M. M. Ultrastructural changes in the surface layers of the newt's egg in relation to the mechanism of its cleavage. J Cell Sci. 1970 Jan;6(1):207–227. doi: 10.1242/jcs.6.1.207. [DOI] [PubMed] [Google Scholar]
- Sheridan J. D. Electrophysiological evidence for low-resistance intercellular junctions in the early chick embryo. J Cell Biol. 1968 Jun;37(3):650–659. doi: 10.1083/jcb.37.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C., Palmer J. F. The permeability of intercellular junctions in the early embryo of Xenopus laevis, studied with a fluorescent tracer. Exp Cell Res. 1969 Jun;55(3):416–419. doi: 10.1016/0014-4827(69)90577-1. [DOI] [PubMed] [Google Scholar]
- Slack C., Warner A. E., Warren R. L. The distribution of sodium and potassium in amphibian embryos during early development. J Physiol. 1973 Jul;232(2):297–312. doi: 10.1113/jphysiol.1973.sp010271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C., Wolpert L. Absence of intracellular potential gradients in amphibian embryos. Nat New Biol. 1972 Apr 5;236(66):153–155. doi: 10.1038/newbio236153a0. [DOI] [PubMed] [Google Scholar]
- Tarin D. Histological features of neural induction in Xenopus laevis. J Embryol Exp Morphol. 1971 Dec;26(3):543–570. [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Tomita T. Membrane capacity and resistance of mammalian smooth muscle. J Theor Biol. 1966 Nov;12(2):216–227. doi: 10.1016/0022-5193(66)90114-7. [DOI] [PubMed] [Google Scholar]
- Warner A. E. Electrical connexions between cells at neural stages of the axolotl. J Physiol. 1970 Sep;210(2):150P–151P. [PubMed] [Google Scholar]
- Warner A. E. The electrical properties of the ectoderm in the amphibian embryo during induction and early development of the nervous system. J Physiol. 1973 Nov;235(1):267–286. doi: 10.1113/jphysiol.1973.sp010387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartiovasra J., Lehtonen E., Nordling S., Saxen L. Do membrane filters prevent cell contacts? Nature. 1972 Aug 18;238(5364):407–408. doi: 10.1038/238407a0. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969 Oct;25(1):1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]


