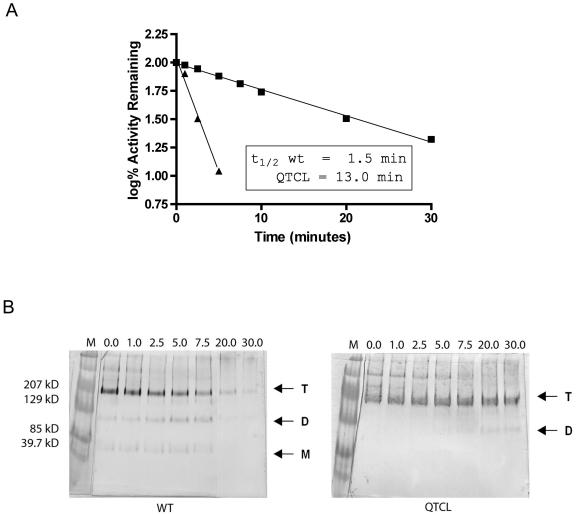
Figure 5.
Heat stability of purified QTCL at 42°C. Enzymes were placed in a water bath at 42°C for varying times and then cooled on ice. Each enzyme was assayed for 10 min in the forward direction in the presence of 10 mm 3-PGA. Reactions were started with 0.065 μg of enzyme. Data were plotted as log% activity versus time (min), and the inactivation constant t1/2 was calculated as follows: slope = −k/(2.3). t1/2 is calculated from the equation k = 0.693/t1/2. A, Inactivation time course at 42°C. Symbols are as follows: ▪, QTCL mutant; and ▴, wild-type enzyme. B, BN-PAGE gel transferred and probed with small subunit antibody at the time points from A. T, The approximately 220-kD tetramer; D, the approximately 100-kD dimer; and M, the approximately 50-kD monomer.