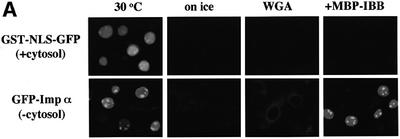
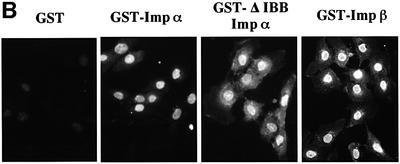
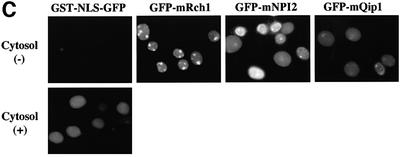
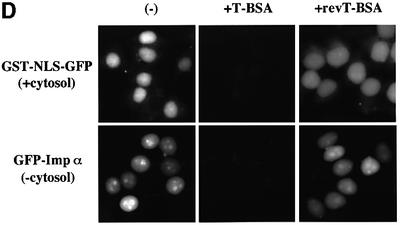
Fig. 2. Importin α is able to migrate into the nucleus in an importin β-independent manner in an in vitro assay. (A–D) Cells were treated with 40 µg/ml digitonin in TB (see Materials and methods) for 5 min on ice, and after washing with PBS twice, the cells were incubated with 10 µl of testing solution. (A) Digitonin-permeabilized MDBK cells were incubated with 2.5 µM GFP–importin α (mRch1) with TB alone or 2.5 µM GST–NLS–GFP with cytosolic extracts prepared from mouse Ehrlich ascites tumor cells and an ATP regeneration system for 20 min at 30°C or on ice. The other import reactions of GFP–importin α or GST–NLS–GFP were performed for 20 min at 30°C after pretreatment with 0.4 mg/ml WGA for 10 min at 30°C, or in the presence of 25 µM MBP–IBB for 20 min at 30°C. (B) Digitonin-permeabilized MDBK cells were incubated with 1 µM wild-type GST–importin α (NPI1) or 1 µM GST–ΔIBB importin α (NPI1; 78–534 amino acids) for 20 min at 30°C. As a control, 1 µM GST alone or 1 µM GST–importin β was used. To detect the GST portion, anti-GST–antibody (B-14; a mouse monoclonal IgG; Santa Cruz Biotechnology, Inc.) (2 µg/ml) was used and detected with RITC-conjugated goat anti-mouse IgG. (C) Digitonin-permeabilized MDBK cells were incubated with 2.5 µM GFP–mRch1, 2.5 µM GFP–mNPI2 and 2.5 µM GFP–mQip1 alone or 2.5 µM GST–NLS–GFP in the presence or absence of Ehrlich cytosolic extracts and an ATP regeneration system for 20 min at 30°C. (D) Digitonin-permeabilized MDBK cells were incubated with 2.5 µM GFP–importin α (mRch1) alone or 2.5 µM GST–NLS–GFP with Ehrlich ascites tumor cells cytosolic extracts and an ATP regeneration system in the presence of 25 µM T-BSA or 25 µM revT-BSA for 20 min at 30°C.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.