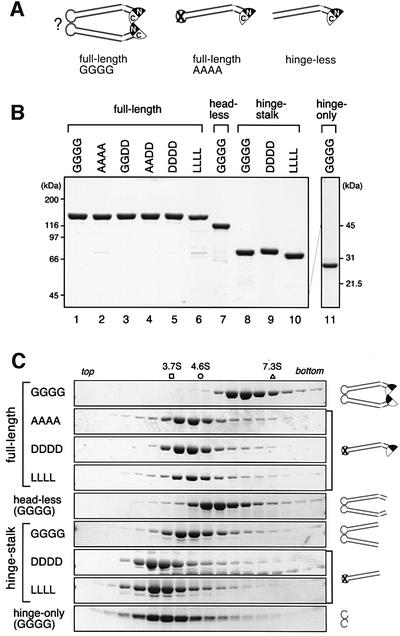
Fig. 1. Design and characterization of BsSMC mutants. (A) Wild-type BsSMC (GGGG) and its mutant derivatives (AAAA and hinge-less) used in a previous study (Hirano et al., 2001). In this diagram, it is postulated that dimerization of BsSMC is mediated by a hinge–hinge interaction (shown by ?). (B) The wild-type and mutant BsSMC proteins were purified, fractionated by SDS–PAGE and stained with Coomassie Blue. (C) The purified BsSMC proteins were fractionated by centrifugation on 5–20% sucrose gradients. Fractions were resolved by SDS–PAGE and stained with Coomassie Blue. The positions of three protein standards [ovalbumin (3.7S), BSA (4.6S) and aldolase (7.3S)] are indicated. The predicted structure for each construct is shown on the right.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.