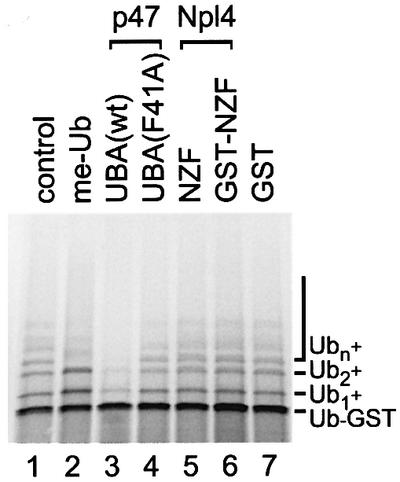
Fig. 6. The UBA domain of p47, but not the zinc finger domain of Npl4, inhibits polyubiquitylation of Ub–GST in vitro. Ub–GST was expressed in a rabbit reticulocyte lysate in the absence or presence of methylated ubiquitin (me-Ub, 20 µM) or 35 µM of the indicated peptides: p47-UBA(wt), p47-UBA(F41A), Npl4-NZF peptide, a GST–NZF fusion or GST. Samples were fractionated using SDS–PAGE and visualized using a phosphoimager. The radiolabeled Ub–GST fusion protein was polyubiquitylated under control conditions (Ub1–n) by factors present in the lysate. Note that p47-UBA(wt) significantly inhibited polyubiquitylation.
