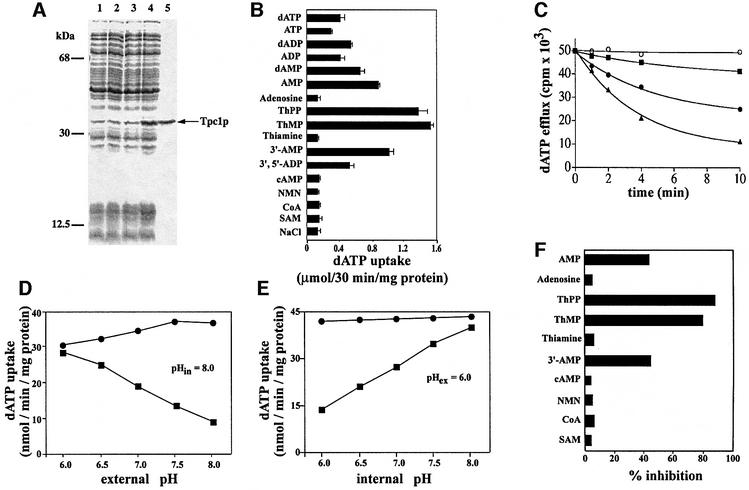
Fig. 1. Tpc1p catalyzes the transport of ThPP and ThMP. (A) Expression of yeast Tpc1p in E.coli. Proteins were separated by SDS–PAGE and stained with Coomassie Blue. The positions of the markers (bovine serum albumin, carbonic anhydrase and cytochrome c) are shown on the left. Lanes 1–4: E.coli CO214(DE3) containing the expression vector with (lanes 2 and 4) and without (lanes 1 and 3) the coding sequence of Tpc1p. Samples were taken at the time of induction (lanes 1 and 2) and 5 h later (lanes 3 and 4). The same number of bacteria was analyzed in each sample. Lane 5: purified Tpc1p (2 µg) originating from bacteria shown in lane 4. (B) Substrate specificity of Tpc1p. Proteoliposomes were pre-loaded internally with various substrates (concentration, 10 mM). Transport was started by external addition of 0.2 mM [α-35S]dATP and stopped after 30 min. (C) Efflux of [α-35S]dATP from proteoliposomes reconstituted with Tpc1p. Proteoliposomes were reconstituted in the presence of 1 mM dATP, 30 mM MES/30 mM HEPES (buffer A) at pH 6.0. The internal substrate pool was labeled by carrier-mediated exchange equilibration. Then the proteoliposomes were passed through Sephadex G-75 columns pre-equilibrated with 50 mM NaCl and 0.1 mM MES/0.1 mM HEPES pH 6.0. The efflux of [α-35S]dATP was started by adding buffer A at pH 6 (filled squares), buffer A at pH 8.0 (filled circles), buffer A at pH 8.0 with 20 mM bathophenanthroline and 60 µM p-CMBS (open circles) or buffer A at pH 8.0 with 10 mM ThPP (filled triangles). (D and E) Dependence on trans-membrane pH gradient of the dATP uniport by Tpc1p. The reconstitution mixture contained 10 mM dATP (dATP/dATP exchange) or 10 mM NaCl (dATP uniport), and buffer A at pH 8.0 (D) or at various pH values from 6.0 to 8.0 (E). After reconstitution of Tpc1p into liposomes, a mixture of 50 mM NaCl and 0.1 mM MES/0.1 mM HEPES at pH 8.0 (D) or various pH values from 6.0 to 8.0 (E) was used to equilibrate and to elute the Sephadex G-75 columns. Transport was started by adding 1 mM [α-35S]dATP together with buffer A at pH values from 6.0 to 8.0 (D) or at pH 6.0 (E). The reaction was terminated after 3 min: dATP uniport (filled squares), dATP/dATP exchange (filled circles). Similar results were obtained in three independent experiments. (F) Effect of externally added substrates on the uptake of [α-35S]dATP into proteoliposomes reconstituted with recombinant Tpc1p. Transport was started by adding 0.2 mM [α-35S]dATP to proteoliposomes containing 10 mM NaCl and no substrate, and stopped after 3 min. All substrates were added together with [α-35S]dATP at a final concentration of 0.8 mM. The extents of inhibition (%) from a representative experiment are reported.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.