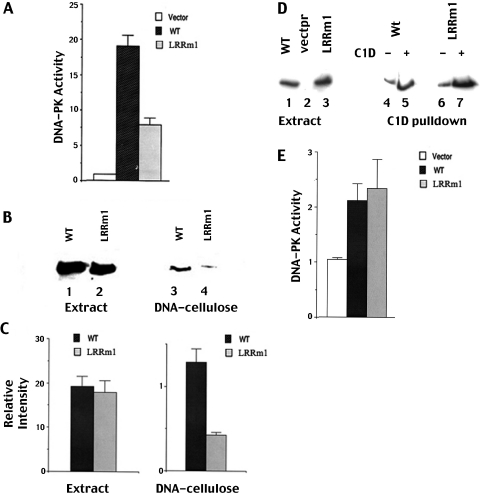
Figure 4.
LRRm1 binds poorly to DNA-cellulose but has normal enzymatic activity. (A) Whole-cell extracts (250 µg) from V3 cells transfected with either vector alone, wild-type DNA-PKcs or LRRm1 were assayed for DNA-PK activity as described in Materials and Methods. Each cell extract was tested in duplicate and three independent extracts were tested for each cell line. (B) Immunoblot analysis of whole cell extracts (500 µg) and DNA-cellulose fractions as indicated from V3 transfectants expressing wild-type DNA-PKcs (lanes 1 and 3) and LRRm1 (lanes 2 and 4). (C) Densitometric analyses of immunoblots from three independent experiments assessing DNA-cellulose fractionation of wild-type DNA-PKcs and LRRm1. Error bars, standard error of the mean. (D) Immunoblot analyses of Ni+ agarose fractions of whole cell extracts (2 mg) from V3 transfectants expressing either wild-type DNA-PKcs (lanes 1, 4 and 5) or LRRm1 (lanes 3, 6 and 7) incubated with whole cell lysates from either control virus infected-Sf9 cells or His-C1D-infected Sf9 cells as indicated. (E) DNA-PKcs absorbed onto C1D that was immobilized onto Ni+-agarose beads was assayed for enzymatic activity as described in Materials and Methods. Three independent extracts were tested for each cell line. Error bars, standard error of the mean.