Figure 6.
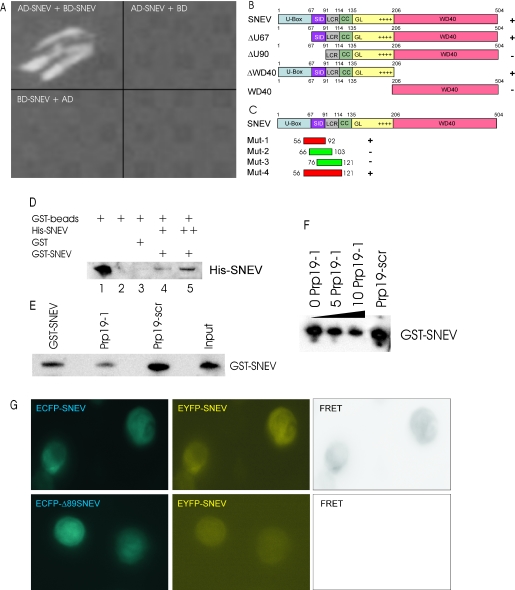
SNEV forms homo-oligomeres that are dependent on the amino acids 56–92 of SNEV. (A) SNEV interacts with SNEV in a yeast two-hybrid assay when GAL4-AD-SNEV and GAL4-BD-SNEV are co-transformed into the yeast reporter strain AH109 (AD-SNEV + BD-SNEV). Neither co-transformation of SNEV fusion to the GAL4 activating domain (AD-SNEV + BD) nor to the DNA-binding domain (BD-SNEV + AD) together with the respective empty second plasmid allowed formation of yeast colonies on high stringency drop-out media plates. (B) Domain mapping of the self interacting amino acids was performed using the Yeast two-hybrid system. Various truncated SNEV constructs were used as prey. Growth of double transformants on high stringency media plates was observed only for variants containing amino acids 68–90, which we therefore termed self interaction domain (SID). (C) In order to confirm that the SID is sufficient for homo-oligomerization, four small overlapping peptides were cloned and tested by Y2H. Co-transformation with SNEV resulted in growth of yeast double transformants on high stringency media plates only with the region 56–92 and 56–121 (red), while neither 66–103 nor 76–121 (green) allowed yeast colony formation. U-box: domain necessary for ubiquitin E3 ligase activity of SNEV; SID: Self Interaction domain; LCR: low complexity region; GL2: globular domain 2, WD40: domain containing 7 WD40 repeats; numbers indicate the amino acid positions, ‘+’ indicates colony formation on high stringency drop out media, ‘−’ indicates no colony formation. (D) The SNEV self-interaction was confirmed by GST pull-down experiments using affinity purified His6–SNEV and GST–SNEV. While no His6–SNEV was detectable in the controls using either GST or beads alone (lanes 2 and 3), GST–SNEV results in co-precipitation of His6–SNEV (lanes 4 and 5). (E) Addition of Prp19-1 peptide to GST–SNEV reduces the amount of precipitated SNEV (Prp19-1), whereas addition of the scrambled control peptide (Prp19-scr) does not. This amount is comparable to the one without addition of peptides (GST–SNEV) as well as to the amount of GST–SNEV added to the beads (Input). (F) Increasing amounts of the Prp19-1 peptide (0, 5 and 10 nM) decrease the amount of precipitated GST–SNEV (G) Additional confirmation of SNEV self interaction derives from FRET analysis. Upper panel: Co-transfection of ECFP–SNEV and EYFP–SNEV into HeLa cells resulted. Pictures were taken using CFP-filter (ECFP–SNEV) displayed in cyan, YFP-filter (EYFP–SNEV). FRET represents the calculated net FRET signal. Lower panel: negative control using ECFP–SNEVΔ98 lacking the interaction domain.