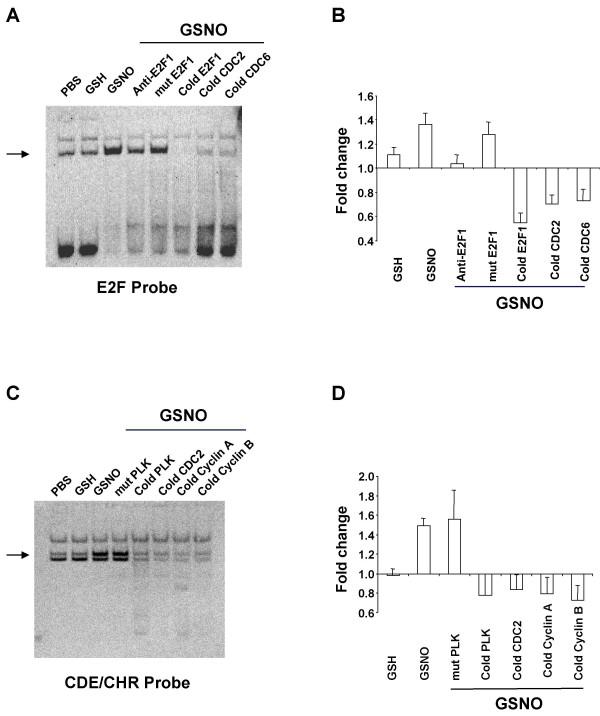
Figure 7.
NO• increases protein binding to E2F and CDE/CHR promoter sites. (A) Representative gel for protein binding to the E2F probe detected by ECL. (B) Results for the E2F probe quantified with laser densitometry. Data, presented as ratios relative to PBS values (set to 1), are means ± SE of six independent experiments. (C) Representative gel for protein binding to CDE/CHR probe detected by ECL. (D) Results for the CDE/CHR probe quantified with laser densitometry. Data, presented as ratios relative to PBS values (set to 1), are means ± SE of six independent experiments. Differentiated U937 cells were incubated with glutathione (GSH; 400 μM) or S-nitrosoglutathione (GSNO; 400 μM) for 3 h. Nuclear extract (15 μg) was prepared and incubated with double-stranded, biotin-N4-CTP labeled DNA probe representing the E2F or CDE/CHR concensus sequence from the E2F1 or PLK promoter, respectively. Protein binding was then determined by electrophoretic mobility shift assay. Complexes were competed with 100-fold molar excess of cold probes, site-directed mutagenesis of the consensus sequence, and for the E2F probe, E2F1-directed antibody as indicated to test for binding specificity.