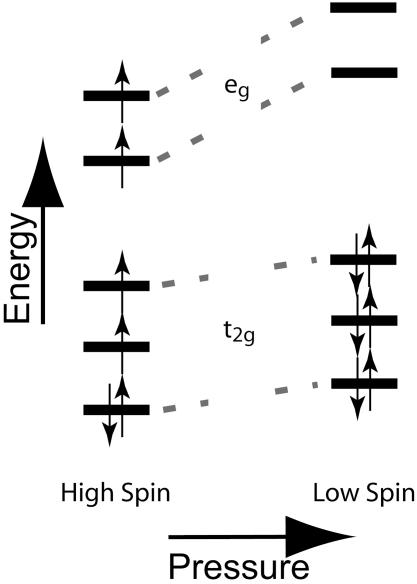
Fig. 1.
Distribution of electrons among 3d orbitals for 6-fold coordinated Fe2+ in high-spin (left) and low-spin (right) configurations. The electronic structure of the ion consists of six 3d electrons around an argon core [1s2 2s2 2p6 3s2 p6], with the eg orbitals pointing toward and the t2g orbitals pointing between the first-neighbor oxygen ions. Hund's rule, predicting that the high-spin state is favored because spin-pairing costs energy, applies at low pressure and results in the ferrous ion having a magnetic moment caused by the presence of unpaired spins. With increasing pressure, the energies of all of the orbitals rise, but they rise more rapidly for the eg than for the t2g because the former experience more extensive overlap with the nearest-neighbor oxygen electrons than do the t2g orbitals. Therefore, the low-spin configuration that is diamagnetic (all electrons being spin-paired, therefore no magnetic moment) becomes energetically favored with pressure. The energies of the two orbital types are reversed for tetrahedral (4-fold), cubic (8-fold), or dodecahedral (12-fold) coordination, with e levels lower than t levels, such that a magnetic moment is present for both high- and low-spin configurations in these cases (3).