Fig. 5.
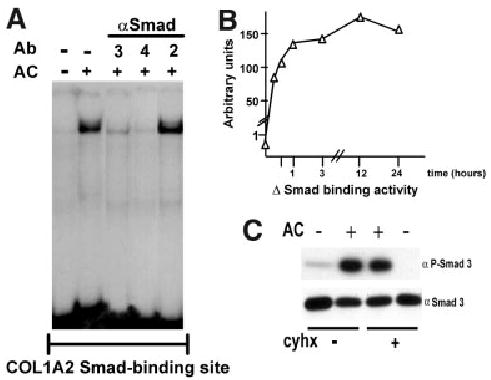
Acetaldehyde enhances phosphorylation of Smad 3 and binding of a Smad3/4-containing complex to the −272 to −249 COL1A2 region by a protein synthesis–independent mechanism. (A) Electrophoretic mobility shift assay performed with nuclear extracts from control and acetaldehyde (AC)-treated HHSCs for 6 hours, using an oligonucleotide containing 3 copies in tandem of the COL1A2 Smad binding site (CAGA)3 as probe. Antibody interference assays with antibodies (Ab) to either Smads 2, 3, or 4 revealed that the complex contained Smads 3/4 but not Smad 2. (B) Time-course analysis of the effect of acetaldehyde on the binding of the Smad 3/4-containing complex to the (CAGA)3 oligonucleotide. The graph was constructed with values obtained after densitometric analysis of bands generated in duplicate electrophoretic mobility shift assays performed at the indicated times and expressed as arbitrary units. (C) Western blot analysis of nuclear extracts obtained from control and acetaldehyde (AC)-treated HHSCs for 6 hours in the presence or absence of cycloheximide (cyhx). Samples were probed with an anti-Smad3 antibodies directly (αSmad3) or after immunoprecipitation with an anti-phosphoserine antibody (αP-Smad3).
