Abstract
The objective of this study was to determine if the immune responses could be differentially modulated by the phytoestrogen genistein (GEN) in mice from the first and second litters, and if the effects were persistent or reversible. B6C3F1 mice were exposed to a control or GEN-containing diet at 25, 250 and 1250 μg/g for the first litters, and 500 μg/g for the second litters from day 0 of gestation to PND22, and through feed after weaning. At PND42, an increase in the anti-CD3 antibody-stimulated splenic T cell proliferation and the percentages of T cells was observed in mice from the first litters at 250 and 1250 μg/g GEN but not from the second litters. At PND84, the activity of IL-2-treated NK cells was significantly increased by GEN in mice from the second litters but not from the first litters. The activity of cytotoxic T cells (CTLs) was also significantly increased by GEN in male mice from the second litters. However, the increases in the CTL activity were not significant when the male mice were shifted from GEN-containing food to control food at PND22. Additionally, the increases in T-cell activities in female mice from the first litters and male mice from the second litters were associated with a decrease in the percentage of CD4+CD25+ T regulatory cells. Overall, the results demonstrated that GEN could enhance the immune responses in mice from the first and second litters; however, the effects varied depending on the exposure duration, gender, and litter order.
Keywords: genistein, developmental exposure, immune stimulation and litter order
Abbreviations: CTLs, cytotoxic T cells; DES, diethylstilbestrol; ER, estrogen receptors; E:T, effector:target ratio; F1M = F1 males; F1F = F1 females; FITC, fluorescein isothiocyanate; GD, gestation day; GEN, genistein; mAb, monoclonal antibody; NCTR, the National Center for Toxicological Research; PND, postnatal day; PE, phycoerythrin
INTRODUCTION
Genistein (GEN), a major isoflavone in most soy products, has been demonstrated to interact with estrogen receptors in vivo. In vitro, it has also been utilized as a tyrosine kinase inhibitor (1, 2). Despite the hypothesized beneficial effects of GEN (e.g., decreased incidences of some hormone-related cancers), there are concerns about the potential long-term effects of this compound on human health, especially that of infants and young children (3). Infants fed soy milk formulas have plasma isoflavone levels that are orders of magnitude higher than those of infants fed human or cows’ milk (4). The possible long-term effects of these relatively high levels of phytoestrogens during infancy are unknown. Epidemiological studies indicate that there is an increase in the use of asthma or allergy drugs in young adults who were fed soy formula during infancy as compared to those who were fed cow milk formula (5). Additionally, phytoestrogens have been detected in amniotic fluid (6–8), suggesting that in utero exposure also occurs.
Our previous studies have demonstrated that exposure to GEN (0 – 20 mg/kg) in adult female B6C3F1 mice for 28 days by gavage increased the activities of cytotoxic T cells (CTLs) and natural killer (NK) cells (9). Additionally, increased splenic T cell number was observed in male and female Sprague Dawley rats when the rats were exposed to GEN gestationally and lactationally by feeding the dams with GEN containing diet (0 – 1250 μg/g), and via the diet from postnatal day (PND) 22 to PND64 (10). Importantly, exposure of experimental animals to natural estrogens such as estradiol or synthetic estrogenic compounds such as diethylstilbestrol (DES) has also been associated with immunotoxic effects, particularly when administered perinatally during lymphoid organ organogenesis (11–13). These effects include thymic atrophy, myelotoxicity, and suppression of NK cell activity.
It has been reported that the immune responses such as the IgE hypersensitivity reaction are affected by birth order in humans. Matricardi et al. (14) have shown that the prevalence of atopy is much higher among first-born children, and that there is a 3% decrease in prevalence for each additional younger sibling. A significant association between the proliferation of cord blood mononuclear cells to house dust mite and birth order was also reported (15). In addition, there is evidence that the time of exposure to GEN predetermines GEN’s biological effects. For example, mammary cancer chemoprevention was demonstrated after prepubertal and combined prepubertal and adult genistein treatments but not after prenatal- or adult-only treatments in rats (16).
The objective of present study was to further evaluate if the effects of GEN on the immune system in B6C3F1 mice were consistent with its estrogenic properties. Depue et al. (17) have suggested that the concentration of free estrogen may be higher in first pregnancies due to a deficit in sex-steroid binding globulin production. It was hypothesized that immune responses could be differentially modulated by GEN in male and female mice from the first and second litters, and the effects might be persistent or reversible depending on the sex. In this study, we have evaluated the effects of GEN on the immune responsiveness in F1 generation (B6C3F1) of female C57BL/6 × male C3H mice. Exposure to GEN occurred through dams gestationally and lactationally, and from feed starting at the weaning day (PND 22) until PND 42 or PND 84.
MATERIALS AND METHODS
Animals and treatments
Both female C57BL/6 and male C3H mice (8–12 weeks old) were obtained from Charles River Breeding Laboratories (Raleigh, NC). To obtain second litter B6C3F1 mice, time-mated primiparous C57BL/6 mice (×male C3H mice) at gestation day (GD) 14 were purchased; and pups were removed from these mice after they gave birth. Two weeks later, time-mated C57BL/6 mice in their second pregnancy were generated through housing two of these female C57BL/6 mice and one male C3H mice in one cage (plug date = gestational day 0), and the pups from these mice in their second pregnancy were considered the second litters. Age-matched time pregnant C57BL/6 mice in their first pregnancy were generated similarly through housing two virgin female C57BL/6 mice and one male C3H mice in one cage, and the pups from these mice in their first pregnancy were considered the first litters. Pregnant mice were housed individually in standard plastic cages with hardwood chip bedding. The female mice were randomized into different treatment groups on the first day of each study, and each group was provided with one of the treatment diets described below one week before the mating and water ad libitum. Each treatment group consisted of 4–8 mice. The animal room was maintained within a temperature range of 22–25°C and relative humidity of 50 ± 20 with 12-h light cycles (7:00–19:00). After parturition, the offspring were housed together with their respective dams, one litter per cage, until weaning on PND 22, at which time the offspring were housed up to four same-sex littermates per cage. To eliminate litter effect, one mouse from each litter for each sex was randomly selected for evaluation.
The diet in a powdered form (5K96, purchased from Purina Mills, St. Louis, MO) is a natural ingredient diet, formulated to be used in experimental protocols where dietary estrogenic activity is a concern. It meets the nutrient specifications as shown for NIH-31 in the 1996 update, except that casein replaces the protein contributed by soy and alfalfa, soy oil is replaced by corn oil, and the vitamin mix is adjusted for irradiation (18). The control diet was assayed for genistein and daidzein after hydrolysis of conjugates. The concentrations of both genistein and daidzein of this diet were determined by LC-ES/MS/MS to be approximately 0.5 μg/g (19). GEN with purity greater than 99% was mixed into the standard 5K96 feed every three months by the Diet Preparation Staff, Bionetics at the National Center for Toxicological Research (NCTR, Jefferson, AR); each dosed batch of feed was analyzed by the Division of Chemistry, and it was stable for at least six months when stored refrigerated.
The dams consumed 5K96 chow containing 0 – 1250 μg/g GEN (Toronto Research Chemicals, North York, Ontario, Canada) starting from approximately two weeks prior to mating to postpartum day 22. One important modification in the experimental design for the second litter study was that only one concentration of GEN was used. The rationale was that the concentration of 500 μg/g was in the range (250–1250 μg/g) in which most of effects were observed in the first litter studies. The F1 mice were exposed to GEN gestationally and lactationally, and to GEN-containing feed from the day of weaning (PND22) to PND42 or PND84. On PND42 and PND84, mice were sacrificed by CO2 inhalation, and the spleens and thymuses were collected for immunological evaluations. All animal procedures were conducted under an animal protocol approved by the VCU Institutional Animal Care and Use Committee (IACUC).
Determination the numbers of thymocytes and splenocytes
The quantification of immune cells was performed as previously described (20). Briefly, splenocytes and thymocytes were prepared by mashing the spleens and thymuses between two slides with frosted ends. After washing, the cells were resuspended in a RPMI complete medium and counted using a Coulter Counter ZII with the red blood cells lysed using a ZAP-OGLOBIN II lytic reagent (Coulter Corporation, Miami, Florida).
Flow cytometric analysis of splenocytes
To determine the percentages of splenocyte subsets, the respective cell types were labeled with an appropriate monoclonal antibody (mAb), conjugated with a fluorescent molecule for visualization as previously described (20). All the antibodies were obtained from BD PharMingen (San Diego, CA). For CD4+ cells, a phycoerythrin (PE) conjugated mAb specific for the L3T4 cell surface protein was used, and for CD8+ cells, a fluorescein isothiocyanate (FITC) conjugated mAb specific for the CD8a (Ly-2) marker was used. For NK cells, a FITC conjugated anti-mouse CD3e mAb and a PE labeled anti-NK1.1 antibody were used. Additionally, FITC anti-mouse CD25 and IgM, and PE anti-mouse CD3 were also used to label splenocytes. Isotype-matched irrelevant antibodies were used as controls. Following the addition of the reagents, the cells were incubated at 4°C in the dark for at least 30 min. After incubation, the cells were washed 2X and propidium iodide (PI) was added as a viability stain. Following incubation with PI for 5 min, the cells were washed and enumeration performed on a Becton Dickinson FACScan Flow Cytometer. Nonviable cells were eliminated through setting a live gate excluding red fluorescence emerging from PI; a forward scatter threshold was set high enough to eliminate red blood cells. For each sample, 5,000 PI negative events were counted.
Natural killer cell activity
Our previous study suggested that GEN affected the IL-2 augmented NK cell activity (9), thus, similar assays were performed in this study. The activity of NK cells was assayed as described using 51Cr -YAC-1 cells (21).
Anti-CD3 antibody-mediated spleen T cell proliferation
The proliferation of splenocytes in the presence of anti-CD3 antibody was performed as previously described (9). The incorporation of 3H-thymidine into the proliferating cells was used as the endpoint of the assay, and the data were expressed as kBq/2 × 105 cells.
Cytotoxic T lymphocyte (CTL) activity
The assay for CTL activity was performed as previsouly described using 51Cr-labeled P815 cells (9). Controls for spontaneous and maximum release were generated by culturing labeled target cells in the presence of either E-MEM medium or 0.1% Triton X-100, respectively.
Statistical analysis
Data are expressed as means ± standard error of means. Results were tested for variance homogeneity using Bartlett's Test. Homogeneous data were analyzed using a one-way analysis of variance; when significant, Dunnett's t Test was used to determine differences between the experimental and vehicle control group. For non-homogeneous data, a non-parametric analysis of variance was used; when significant, differences between the control and experimental groups were determined by the Wilcoxon Rank Test. The Jonckheere’s Test was used to determine an exposure level-related trend. Male and female mice were analyzed separately. P values of 0.05 or less were considered statistically significant.
RESULTS
GEN on the body weight and organ weights
Exposure to GEN produced a significantly decreased terminal body weight in the first litter males at the levels of 25 μg/g and above and in the first litter females at the levels of 25 and 1250 μg/g at PND42 (Table 1). The decreases in terminal body weight were still observed in adult (PND84) first litter male mice at the levels of 250 and 1250 μg/g and female mice at 1250 μg/g (Table 2). However, no decrease in the terminal body weight was observed in the second litter male and female mice at 500 μg/g GEN at either PND42 or PND84 (Table 1 and 2).
TABLE 1.
Effect of genistein exposure form GD0 to PND42 on terminal body weight and organ weights in B6C3F1 mice1
| Spleen Weight
|
Thymus Weight
|
||||||
|---|---|---|---|---|---|---|---|
| Genistein (μg/g) | n2 | Body Weight (g) | mg | g/100 g body | mg | g/100 g body | |
| First litter | |||||||
| F1M | Control | 8 | 18.0 ± 0.3 | 72.9 ± 2.2 | 0.41 ± 0.01 | 69.4 ± 3.3 | 0.39 ± 0.02 |
| 25 | 8 | 16.1 ± 0.5* | 69.3 ± 2.6 | 0.43 ± 0.02 | 72.0 ± 3.4 | 0.45 ± 0.02 | |
| 250 | 7 | 14.9 ± 0.7* | 77.1 ± 4.7 | 0.53 ± 0.04* | 73.1 ± 1.5 | 0.50 ± 0.03* | |
| 1250 | 8 | 13.7 ± 0.7** | 67.5 ± 3.6 | 0.50 ± 0.02* | 77.3 ± 4.1 | 0.58 ± 0.04** | |
| F1F | Control | 8 | 15.3 ± 0.4 | 67.6 ± 1.9 | 0.44 ± 0.01 | 83.1 ± 3.3 | 0.54 ± 0.02 |
| 25 | 6 | 13.8 ± 0.4* | 68.8 ± 3.2 | 0.53 ± 0.04* | 80.5 ± 2.4 | 0.60 ± 0.03 | |
| 250 | 8 | 13.9 ± 0.8 | 67.3 ± 4.6 | 0.51 ± 0.06 | 84.3 ± 4.4 | 0.63 ± 0.06 | |
| 1250 | 8 | 12.5 ± 0.4* | 65.4 ± 2.5 | 0.53 ± 0.03* | 74.4 ± 4.3 | 0.60 ± 0.04 | |
| Second litter | |||||||
| F1M | Control | 5 | 15.9 ± 0.4 | 71.4 ± 6.0 | 0.45 ± 0.03 | 57.6 ± 5.4 | 0.36 ± 0.03 |
| 500 | 5 | 15.8 ± 0.5 | 67.4 ± 4.0 | 0.43 ± 0.03 | 63.8 ± 4.1 | 0.41 ± 0.04 | |
| F1F | Control | 5 | 14.2 ± 0.3 | 64.4 ± 2.6 | 0.45 ± 0.02 | 85.4 ± 1.8 | 0.60 ± 0.02 |
| 500 | 5 | 14.0 ± 0.2 | 64.8 ± 4.1 | 0.46 ± 0.03 | 75.2 ± 8.3 | 0.54 ± 0.06 | |
Values represent the means ± SE. F1M = F1 males, F1F = F1 females. Asterisks indicate a difference from the control:
p ≤ 0.05,
p ≤ 0.01.
n = the number of mice in each group.
TABLE 2.
Effect of genistein exposure from GD0 to PND84 on terminal body weight and spleen weights in B6C3F1 mice1
| Spleen Weight
|
|||||
|---|---|---|---|---|---|
| Genistein (μg/g) | n2 | Body Weight (g) | mg | g/100 g body | |
| First litter | |||||
| F1M | Control | 7 | 26.7 ± 0.3 | 82.6 ± 2.3 | 0.31 ± 0.01 |
| 25 | 2 | 26.3 ± 0.6 | 84.0 ± 10.0 | 0.32 ± 0.03 | |
| 250 | 5 | 24.0 ± 0.6* | 84.8 ± 2.7 | 0.35 ± 0.01* | |
| 1250 | 5 | 25.4 ± 0.2* | 102.0 ± 3.5* | 0.40 ± 0.01* | |
| F1F | Control | 5 | 20.4 ± 0.4 | 97.4 ± 5.9 | 0.48 ± 0.02 |
| 25 | 6 | 20.7 ± 0.2 | 104.3 ± 6.8 | 0.50 ± 0.03 | |
| 250 | 6 | 18.5 ± 1.1 | 126.7 ± 38.3 | 0.76 ± 0.30 | |
| 1250 | 4 | 18.6 ± 0.2* | 90.0 ± 4.1 | 0.48 ± 0.03 | |
| Second litter | |||||
| F1M | Control | 7 | 25.1 ± 0.4 | 71.6 ± 2.8 | 0.29 ± 0.01 |
| 500 | 7 | 24.2 ± 0.4 | 53.3 ± 4.9* | 0.22 ± 0.02* | |
| 500 To Ctr | 8 | 24.7 ± 0.3 | 53.0 ± 4.6* | 0.21 ± 0.02* | |
| F1F | Control | 8 | 18.2 ± 0.2 | 81.8 ± 8.6 | 0.45 ± 0.05 |
| 500 | 6 | 18.4 ± 0.5 | 78.8 ± 3.7 | 0.43 ± 0.01 | |
| 500 To Ctr | 8 | 19.1 ± 0.3* | 80.5 ± 1.8 | 0.42 ± 0.01 | |
F1M = F1 males, F1F = F1 females. “500 To Ctr” = mice were shifted from GEN diet to control diet at PND22. Values represent the means ± SE. Asterisks indicate a difference from the control:
p ≤ 0.05.
n = the number of mice in each group.
Exposure to GEN from GD0 to PND42 did not affect the absolute spleen weight and thymus weight in either the first litter or second litter mice (Table 1); however, it induced an significant increase in relative spleen weight in both male mice at 250 and 1250 μg/g and female mice at 25 and 1250 μg/g from the first litters but not from the second litter (Table 1). An increase in relative thymus weight was only observed in the first litter male mice at 250 and 1250 μg/g at PND 42 (Table 1). At PND84, exposure to GEN produced an increase in relative spleen weight in the first litter male mice at 250 and 1250 μg/g while a decrease from the second litter male mice at 500 μg/g, and these changes were associated with a corresponding alteration in absolute spleen weight (Table 2). Neither absolute nor relative spleen weights were altered in female mice from either the first litters or the second litters at PND 84 (Table 2).
GEN on the activation of T cells
The proliferative response of splenocytes was evaluated in the presence or absence of anti-CD3 antibody, a T-cell stimulator. At PND42, a dose-related increase in the anti-CD3 antibody-stimulated splenic T cell proliferation was observed in both first litter male and female mice with significant changes observed at the levels of 250 and 1250 μg/g (Figure 1A and 1B). A significant increase in the basal splenocyte proliferation (38.3 ± 7.5 kBq/2 × 105 cells in the treatment group vs. 24.5 ± 1.9 kBq/2 × 105 cells in the control group) was observed in males at 1250 μg/g but not in females (Figure 1A and B). However, neither the anti-CD3 antibody-stimulated nor the basal splenocyte proliferation was altered by GEN at 500 μg/g in the second litter male and female mice (Figure 1C and 1D). To determine if the enhanced T cell proliferation was due to a change in the percentage of T cells, a flow cytometric analysis of T cell population was performed. A significant increase in the percentages of CD3+ T cells was observed in both the first litter male (Figure 2A) and female (Figure 2B) mice at 250 and 1250 μg/g GEN. However, neither the percentage of CD4+ T cells nor that of CD8+ T cells was significantly altered by GEN at 500 μg/g in the second litter male and female mice (data not shown).
Figure 1.
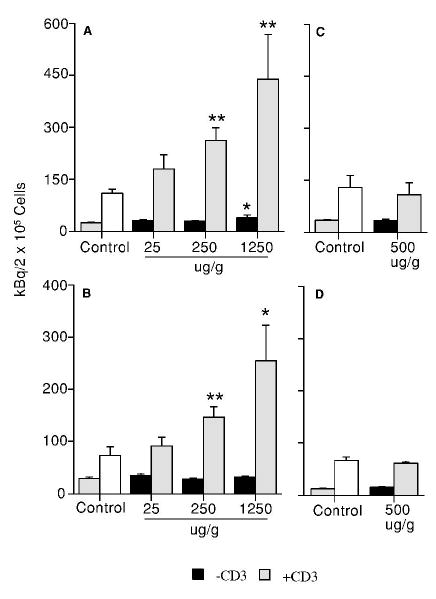
Effect of genistein on spleen cell proliferative response to anti-CD3 antibody stimulation in F1 mice at PND42. (A) Male mice from the first litters; (B) female mice from the first litters; (C) male mice from the second litters; and (D) female mice from the second litters. Mice were exposed to genistein, and anti-CD3 antibody-mediated splenocyte proliferation determined as described in the absence (black bars) or presence (hatched bars) of stimulation. The right-side bars in the control groups are spleen cell proliferative response to anti-CD3 antibody stimulation. Values represent the means ± SE. n = 5–8. Asterisks indicate a difference from the control: *, p ≤ 0.05; **, p ≤ 0.01.
Figure 2.
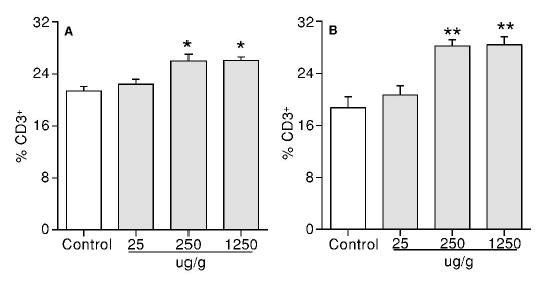
Flow cytometric analysis of splenic T cell populations in F1 mice at PND42. (A) The percentage of CD3+ T cells in male mice from the first litters; (B) the percentage of CD3+ T cells in female mice from the first litters. Values represent the means ± SE. n = 6–8. Asterisks indicate a difference from the control: *, p ≤ 0.05; **, p ≤ 0.01.
At PND84, neither the anti-CD3 antibody-stimulated nor the basal splenocyte proliferation was altered by GEN at 25-1250 μg/g in the first litter male and female mice (data not shown). Although there was no change in the anti-CD3 antibody-stimulated splenic T cell proliferation in the second litter male and female mice, exposure to GEN produced an increase (33%) in the basal splenocyte proliferation in male mice at 500 μg/g GEN. However, this increase was not significant when the male mice were shifted from GEN-containing food to control food after weaning at PND22 (data not shown). A flow cytometric analysis was performed to determine the percentages of splenic T cell populations at PND 84. Although the percentage of CD4+CD8− T cells was decreased by GEN at 1250 μg/g in the first litter females, and that of CD4−CD8+ T cells was decreased at 250 μg/g in the first litter males, there was no significant change in the CD3+ T cell population (Table 3). In the second litter males, exposure to GEN at 500 μg/g produced a significant increase in the percentages of CD3+IgM− T cells and CD4−CD8+ T cells (Table 3). In contrast, a significant increase in the percentages of CD4+CD8− T cells was observed in the second litter females. These increases were not significant when the mice were shifted from GEN-containing food to control food after weaning at PND22 (Table 3).
TABLE 3.
Effect of genistein exposure form GD0 to PND84 on the percentages of differential splenocytes in B6C3F1 mice1
| Genistein (μg/g) | CD3+ | CD4+CD8− | CD4−CD8+ | NK | |
|---|---|---|---|---|---|
| %
|
|||||
| First litter | |||||
| F1M | Control | 21.56 ± 0.87 | 18.65 ± 1.36 | 5.65 ± 0.35 | 2.59 ± 0.13 |
| 25 | 24.97 ± 0.22 | 22.26 ± 3.39 | 4.28 ± 1.38 | 2.78 ± 0.69 | |
| 250 | 22.51 ± 1.09 | 18.87 ± 0.75 | 3.57 ± 0.43* | 2.33 ± 0.28 | |
| 1250 | 21.21 ± 1.93 | 18.40 ± 1.83 | 4.75 ± 0.28 | 2.48 ± 0.28 | |
| F1F | Control | 19.22 ± 1.36 | 17.22 ± 1.02 | 3.30 ± 0.24 | 2.22 ± 0.13 |
| 25 | 21.05 ± 0.77 | 18.47 ± 1.36 | 3.58 ± 0.13 | 2.44 ± 0.13 | |
| 250 | 19.91 ± 1.29 | 15.33 ± 1.46 | 3.93 ± 0.54 | 2.20 ± 0.25 | |
| 1250 | 18.9 ± 0.85 | 13.13 ± 0.51* | 3.61 ± 0.16 | 2.43 ± 0.26 | |
| Second litter | |||||
| F1M | Control | 24.51 ± 1.28 | 17.03 ± 1.25 | 7.66 ± 0.63 | 3.76 ± 0.29 |
| 500 | 27.77 ± 0.74* | 19.31 ± 0.37 | 9.24 ± 0.16* | 3.86 ± 0.05 | |
| 500 To Ctr2 | 26.20 ± 1.33 | 18.36 ± 1.22 | 9.19 ± 0.56 | 4.39 ± 0.15 | |
| F1F | Control | 28.89 ± 2.13 | 16.73 ± 0.98 | 7.04 ± 0.51 | 3.49 ± 0.16 |
| 500 | 30.56 ± 0.97 | 19.84 ± 0.95* | 7.63 ± 0.42 | 4.55 ± 0.18** | |
| 500 To Ctr | 29.67 ± 1.14 | 17.86 ± 0.57 | 7.53 ± 0.31 | 4.01 ± 0.09* | |
Values represent the means ± SE derived from the number of mice as shown in Table 2. F1M = F1 males, F1F = F1 females. Asterisks indicate a difference from the control:
p ≤ 0.05,
p ≤ 0.01.
“500 To Ctr” = mice were shifted from GEN diet to control diet at PND22.
The activity of CTLs was also determined in the second litter B6C3F1 mice at PND84. As shown in Figure 3A, the activity of CTLs was significantly increased by GEN at 500 μg/g at the E:T ratios of 25:1, 12.5:1, 6.25:1 and 3.125:1 in the second litter males. However, these increases were not significant when the male mice were shifted from GEN-containing food to control food after weaning (Figure 3A). No change in the CTL activity was observed in the second litter females at PND84 after GEN exposure from GD0 to PND84 (Figure 3B) or from GD0 to PND22 (Figure 3B).
Figure 3.
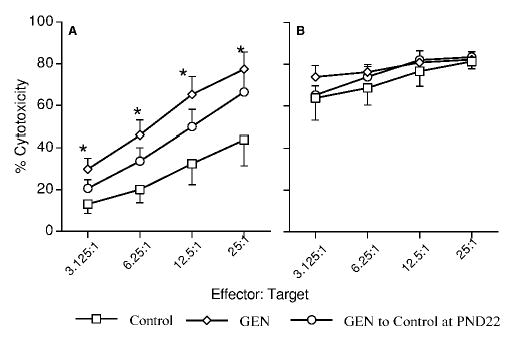
Effect of genistein on splenic CTL activity in F1 mice from the second litters at PND84. Mice were exposed to control or genistein (500μg/g)-containing diet, and the activity of CTLs against P815 cells determined as described. (A) Male mice; (B) female mice. Values represent the means ± SE. n = 6–8. Asterisks indicate a difference from the control: *, p ≤ 0.05.
GEN on NK cell activity
Overall, exposure to GEN had minimal effects on NK cell activity in both male and female mice from the first and second litters at PND42, although there were some increases in the cytotoxic activity in the 250 μg/g-concentration group in female mice from the first litters (Table 4).
TABLE 4.
Effect of genistein exposure form GD0 to PND42 on the activity of NK cells in first litter female B6C3F1 mice1
| Effector: Target
|
||||||
|---|---|---|---|---|---|---|
| 6.25:1 | 12.5:1 | 25:1 | 50:1 | 100:1 | 200:1 | |
| Genistein (μg/g) | %Cytotoxicity | |||||
| Control | 1.76 ± 0.58 | 1.88 ± 0.37 | 3.24 ± 0.50 | 5.23 ± 0.83 | 10.06 ± 1.94 | 17.54 ± 3.05 |
| 25 | 1.84 ± 0.43 | 3.02 ± 1.00 | 3.94 ± 0.53 | 6.69 ± 0.31 | 11.50 ± 0.54 | 22.86 ± 1.63 |
| 250 | 1.49 ± 0.38 | 2.41 ± 0.44 | 5.19 ± 0.60* | 8.77 ± 0.91* | 15.31 ± 1.02* | 26.67 ± 1.15* |
| 1250 | 1.30 ± 0.45 | 1.83 ± 0.42 | 3.49 ± 0.49 | 6.88 ± 1.06 | 12.77 ± 1.29 | 22.77 ± 1.71 |
IL-2-augmented NK cell activity was determined as described. NK cell-specific lysis (%) of 51Cr-labeled YAC-1 cells was used as the endpoint of the assay. Values represent the mean ± SE. n = 6–8. Asterisks indicate a difference from the control:
p ≤ 0.05.
At PND84, exposure to GEN did not produce any significant increases on IL-2-augmented NK cell activity in either the first litter male or female mice (data not shown). In contrast, the activity of IL-2-augmented NK cells in the second litter mice at PND84 was significantly increased by GEN at 500 μg/g (Figure 4A and 4B), and these increases were still significant when the mice were shifted from GEN-containing food to control food after weaning (Figure 4C and 4D).
Figure 4.
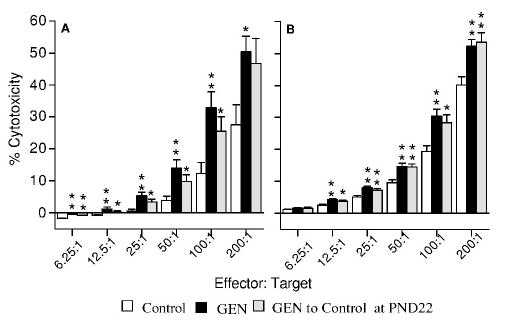
Effect of genistein (500 μg/g) on IL-2-augmented splenic NK cell activity in F1 mice from the second litters at PND84. (A) Male mice exposed to control diet, GEN-containing diet from GD0 to PND84, or GEN-containing diet from GD0 to PND22 and then shifted to control diet; (B) female mice exposed to control diet, GEN-containing diet from GD0 to PND84, or GEN-containing diet from GD0 to PND22 and then shifted to control diet. Values represent the means ± SE. n = 6–8. Asterisks indicate a difference from the control: *, p ≤ 0.05; **, p ≤ 0.01.
Flow cytometric analysis of splenic NK cells (CD3−NK1.1+) revealed that exposure to GEN did not affect the percentages of NK cells in either male or female mice from the first litters at PND 84 (Table 3). The percentage of NK cells was not affected in the second litter males; however, the percentage of NK cells was increased in the second litter females, and this increase was still significant when the female mice were shifted from GEN-containing food to control food after weaning (Table 3).
GEN on the percentages of splenic CD4+CD25+ regulatory T cells`
To explore the possible underlying mechanisms that GEN enhanced the immune responses, a flow cytometric analysis of T regulatory cells (CD4+CD25+) was performed. Exposure to GEN from GD0 to PND42 at 1250 μg/g produced a significant decrease in the percentage of CD4+CD25+ T cells in the first litter female mice but not in the male mice (Figure 5A and 5B). However, exposure to GEN at 500 μg/g from GD0 to PND42 did not change the percentage of CD4+CD25+ T cells in either male or female mice from the second litters (Figure 5C and 5D). Exposure to GEN from GD0 to PND84 at 500 μg/g produced a significant decrease in the percentage of splenic CD4+CD25+ T cells in the second litter male mice (Figure 5E) but not in the female mice (Figure 5F); however, this decrease was not significant (P > 0.05) when the male mice were shifted from GEN-containing food to control food after weaning at PND22 (Figure 5E).
Figure 5.
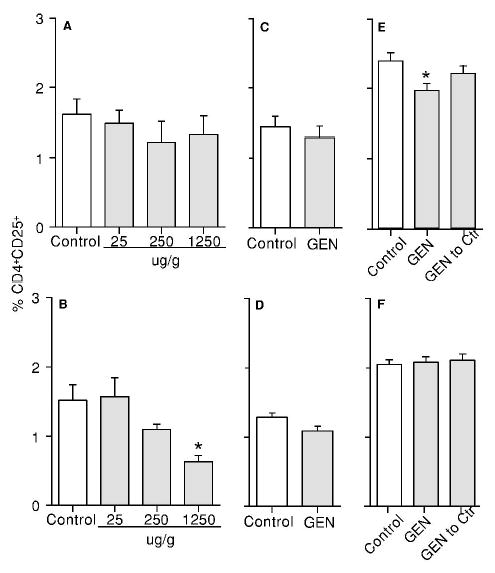
Effect of genistein on the percentage of CD4+CD25+ regulatory T cells in F1 mice. (A) Male mice from the first litters at PND42; (B) female mice from the first litters at PND42; (C) male mice from the second litters at PND42; (D) female mice from the second litters at PND42; (E) male mice from the second litters at PND84; and (F) female mice from the second litters at PND84. “GEN to Ctr” = mice were shifted from GEN (500 μg/g) diet to control diet at PND22. Values represent the means ± SE. n = 5–8. Asterisks indicate a difference from the control: *, p ≤ 0.05.
DISCUSSION
Our previous studies have provided evidence that the immune responses were altered following oral exposure to GEN at physiologically relevant concentrations in experimental animals (9, 10). In this study, we have further examined the effects of dietary GEN on the immune responses in both the first and second litter B6C3F1 mice. Exposure of F1 generation mice was gestational and lactational, and through feeding after weaning at PND22. Although both human and cow’s milk contain low levels of isoflavones (22), isoflavones have been identified in amniotic fluid, suggesting they may pass placental barrier (6–8). For a 25 g mouse consuming 2 g chow every day, the concentrations of GEN at 25, 250, 500 and 1250 μg/g are approximately equivalent to doses of 2, 20, 40 and 100 mg GEN/kg/day, respectively. For a 4-month-old infant who consumes soy formula as directed by the manufacturers, approximately 6–9 mg/kg body weight of isoflavones can be achieved (23).
In this study, the terminal body weights were decreased by GEN, at least at the 250 μg/g concentration and above, in both the first litter male and female mice at PND42 and PND84. However, no changes in terminal body weights were observed in the second litter mice at PND42 and PND84. In our previous studies, decreases in the terminal body weights were also observed in F1 Sprague-Dawley rats born from primiparous dams at 1250 μg/g (10), which might be related to a significant decrease of food intake in this high GEN concentration group by dams (24). However, there was no significant change in food consumption in pregnant mice at GEN levels of 500 μg/g or lower in this study (data not shown). These observations suggested that exposure to GEN might be associated with a general toxicity in the first litter mice but not in the second litter mice. Additionally, the relative weight of the spleen was increased by GEN in the first litter mice at PND42 and PND84 except for the female mice at PND84, which was consistent with the findings that an increase in the relative weight of the spleen was also observed in GEN-exposed Sprague Dawley rats born from primiparous dams when the rats were exposed to GEN-containing feed (0 – 1250 μg/g) gestationally, lactationally and from feeding from GD7 to PND64 (10). In contrast, there was a decrease in the spleen weight in male mice from the second litters at PND84. This decrease might be due to a change in erythrocyte components because there was no significant change in the number of white blood cells in the spleen (data not shown).
Our previous studies have demonstrated that exposure to GEN by gavage increased IL-2-augmented NK cell activity in adult female B6C3F1 mice (9). Interestingly, an increase in the activity of IL-2-activated NK cells in GEN-exposed second litter male and female mice at PND84 was also observed in this study. This observation is also in agreement with our previous report that dietary GEN exposure increased NK cell activity in F0 Sprague-Dawley rats when the animals were exposed from GD7 to postpartum day 51 (10). However, GEN had minimal effect on the activity of NK cells in the first litter male and female mice at PND84, which was consistent with our previous report that GEN had no significant effects on NK cell activity in male and female Sprague Dawley rats born from primiparous dams when the rats were exposed to GEN from GD7 to PND64 (10). Although the exact litter information was not available on the adult female B6C3F1 mice and F0 Sprague-Dawley rats used in our previous studies in which enhanced NK cell activity was observed following GEN exposure (9, 10), it was most likely that these animals were from the second litters and higher, considering that animal suppliers usually breed the dams multiple times to produce more pups.
It should be noted that the increases in NK activity at PND84 in the second litter mice were maintained even after the mice were shifted from GEN diet to control diet at PND22, which suggested that the effect of GEN on NK cells in the second litter mice at PND84 might be due to an imprinting mechanism occurred during developmental exposure since there is evidence that dietary GEN exposure alters methylation patterns in mouse genome (25). The expression of perforin has been shown to be under the regulation of DNA methylation and chromatin remodeling (26). Thus, in the second litter male mice, the increase in NK cell activity might be due to a change in the perforin gene expression because there was no change in the percentage of NK cells associated with the increase of NK activity. In the second litter female mice, the increase in NK cell activity was associated with an increase in the percentage of NK cells. Thus, the mechanisms such as inactivation of the cell cycle regulatory genes (e.g., p16INK4A, p15INK4B, p21Waf1/Cip1, p27Kip1 and p73) by DNA methylation could be involved (27).
In contrast, neither the NK activity in the first litter males nor that in the second litter males was affected by GEN at PND42. Although the NK cell activities in the first litter female mice were slightly increased at PND42 at GEN concentration of 250 μg/g, this increase was not dose-related. The mechanism for an increased response only at the 250 μg/g diet group in first litter females is currently unclear. It might be due to the anti-estrogenic effect of GEN at the 250 μg/g concentration; however, at a higher GEN concentration such as 1250 μg/g, other effects of GEN such as tyrosine kinase inhibition might also be present (2). Additionally, exposure to GEN did not significantly alter the NK cell activities in the second litter female mice at PND42. Therefore, GEN had minimal effect on NK cell activity at PND42 in both the first and second litter mice. In human, the age of 18 years old is approximately corresponding to PND42 in mice (28). Thus, exposure to GEN seemed to have minimal effect on the NK cell activity in early ages.
In this study, the first litter male and female mice exhibited an enhanced anti-CD3 antibody-mediated splenocyte proliferation at PND42, which was partially due to an increase in the percentages of splenic T cells. These increases are also consistent with the observation in Sprague-Dawley rats that the numbers of T cells are increased by GEN when the animals were exposed to GEN from GD 7 to PND 64 (10). Although the anti-CD3 antibody-mediated splenocyte proliferation at PND84 was not significantly altered by GEN in either male or female mice from the first and second litters, an increase in the percentage of CD3+ T cells and CD8+ T cells, and more importantly, an increase in the CTL activity were observed in the second litter male mice at PND84. Thus, the T-cell activities in male mice were modulated by GEN no matter whether the male mice were from the first or second litters. However, the T-cell activities in female mice were modulated by GEN only when the female mice were from the first litters.
The CD4+CD25+ regulatory T cell has been shown to suppress immune responses against foreign antigens and pathogens (29–33). Importantly, exposure to GEN produced a decrease in the percentage of CD4+CD25+ T regulatory cells in the first litter female mice at PND42, the time when an enhanced anti-CD3 antibody-mediated proliferation was observed. A decrease in the percentage of CD4+CD25+ T cells was also observed in GEN-treated second litter male mice at PND84. When the male mice were shifted from GEN diet to control diet at PND22, the percentage of CD4+CD25+ T regulatory cells returned to a level that was comparable to the control mice, and the significance in the increase of CTL activity was also lost. Thus, the effect of GEN on CD4+CD25+ regulatory T cells might be partially responsible for GEN’s stimulatory effect on T cells.
One of the important mechanisms by which GEN exerts its effect on multiple organ systems is to interact with estrogen receptors and competing with estrogen for binding (2). There is evidence that both ERα and ERβ are expressed in NK cells; but ERβ instead of ERα might be responsible for 17β-estradiol-induced suppression of NK activity (34). The expression of ERs has also been reported in CD8+ T cells (12, 35). In contrast to NK cells, ERα but not ERβ is required for 17β-estradiol-induced increases in IFN-γ expression and Th1 responses (36–38). In our studies, both the activities of T cells and NK cells, at least in second litter male mice at PND84, were increased by GEN. It is conceivable that GEN increases NK activity by antagonizing the estrogen’s suppressive effect through ERβ because GEN has 7–20 times higher affinity to ERβ than ERα (39, 40). In contrast, it is unlikely that GEN increases CTL activity by functioning as an estrogen agonist through ERα because GEN’s affinity for ERα is low (39, 40). However, it is possible that binding of GEN to ERβ would leave more free 17β-estradiol to interact with ERα, and thus, T cell activity is enhanced.
The exact mechanism for differential immune stimulation by GEN in B6C3F1 mice from the first and second litters following developmental and adult exposures is currently unknown. An increased level of estrogen has been reported in first pregnancy (17, 41), and this may partially be responsible for the differential immunomodulation in the first and second litters. Additionally, there is evidence that serum level of corticosterone, an immunosuppressive factor, is reduced after GEN administration in rats (42). In first pregnancy, there is an increased level of cortisol in the serum (41), which may be an alternative explanation for the differences in GEN-mediated immunomodulation in the first and second litters. However, the mechanisms mentioned above still could not fully explain the differential effects of GEN on NK activity and T cell activity in male and female mice from the first and second litters.
In conclusion, our results demonstrated that the activities of both NK cells and T cells could be differentially modulated by GEN in male and female mice from the first and second litters during adult and developmental exposures. Furthermore, these effects varied depending on exposure duration, gender and litter order. GEN modulation of immune responses in animals might shed some light on the epidemiological findings (5) that there is an increase in the use of asthma or allergy drugs in young adults who were fed soy formula during infancy as compared to those who were fed cow milk formula. Both NK and T cells contribute significantly to the disease persistence and progression in asthma and allergy (43, 44). Additionally, it would be of value to further examine if the potential of GEN modulating host resistances to tumors is related to its immunomodulatory effect in different periods of life. Thus, further study to determine the susceptibility to develop asthma, autoimmunity, and subsequent autoimmune diseases following GEN exposure is warranted.
Acknowledgments
The authors would like to thank D. L. Musgrove, X. L. Zhang and R. D. Brown for their technical help, and Connie Weis (Division of Biochemical Toxicology, National Center for Toxicological Research, U.S. Food and Drug Administration, Jefferson, AR 72079) for providing GEN feed.
Footnotes
This study was supported by the funds provided from NIH R21ES 012286, and partially from NIEHS contract NO1-ES-05454.
References
- 1.Martin PM, Horwitz KB, Ryan DS, McGuire WL. Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology. 1978;103:1860–1867. doi: 10.1210/endo-103-5-1860. [DOI] [PubMed] [Google Scholar]
- 2.Kurzer MS, Xu X. Dietary phytoestrogens. Ann Rev Nutr. 1997;17:353–381. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- 3.Bingham SA, Atkinson C, Liggins J, Bluck L, Coward A. Phyto-oestrogens: where are we now? Br J Nutr. 1998;79:393–406. doi: 10.1079/bjn19980068. [DOI] [PubMed] [Google Scholar]
- 4.Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 5.Strom BL, Schinnar R, Ziegler EE, Barnhart KT, Sammel MD, Maconesm GA, Stallings VA, Drulis JM, Nelson SE, Hanson SA. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA. 2001;15:807–814. doi: 10.1001/jama.286.7.807. [DOI] [PubMed] [Google Scholar]
- 6.Foster WG Chan S, Platt L Hughes CL., Jr Detection of phytoestrogens in samples of second trimester human amniotic fluid. Toxicol Lett. 2002;129:199–205. doi: 10.1016/s0378-4274(02)00018-8. [DOI] [PubMed] [Google Scholar]
- 7.Adlercreutz H, Yamada T, Wahala K, Watanabe S. Maternal and neonatal phytoestrogens in Japanese women during birth. Am J Obstet Gynecol. 1999;180:737–743. doi: 10.1016/s0002-9378(99)70281-4. [DOI] [PubMed] [Google Scholar]
- 8.Doerge DR, Churchwell MI, Chang HC, Newbold RR, Delclos KB. Placental transfer of the soy isoflavone genistein following dietary and gavage administration to Sprague Dawley rats. Reprod Toxicol. 2001;15:105–110. doi: 10.1016/s0890-6238(01)00108-3. [DOI] [PubMed] [Google Scholar]
- 9.Guo TL, McCay JA, Zhang LX, Brown RD, You L, Karrow NK, Germolec DR, White KL., Jr Genistein modulates immune responses and increases host resistance to B16F10 tumor in adult female B6C3F1 mice. J Nutr. 2001;131:3251–3258. doi: 10.1093/jn/131.12.3251. [DOI] [PubMed] [Google Scholar]
- 10.Guo TL, White KL, Jr, Brown RD, Delclos KB, Newbold RR, Weis C, Germolec DR, McCay JA. Genistein modulates splenic natural killer cell activity, antibody-forming cell response, and phenotypic marker expression in F0 and F1 generations of Sprague-Dawley rats. Toxicol Appl Pharmacol. 2002;181:219–227. doi: 10.1006/taap.2002.9418. [DOI] [PubMed] [Google Scholar]
- 11.Luster MI, Hayes H, Korach TK, Tucker AN, Dean JH, Greenlee WF, Boorman GA. Estrogen immunosuppression is regulated through estrogenic responses in the thymus. J Immunol. 1984;133:110–116. [PubMed] [Google Scholar]
- 12.Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- 13.Holladay SD, Blaylock BL, Comment CE, Heindel JJ, Fox WM, Korach KS, Luster MI. Selective prothymocyte targeting by prenatal diethylstilbesterol exposure. Cell Immunol. 1993;152:131–142. doi: 10.1006/cimm.1993.1273. [DOI] [PubMed] [Google Scholar]
- 14.Matricardi PM, Franzinelli F, Franco A, Caprio G, Murru F, Cioffi D, Ferrigno L, Palermo A, Ciccarelli N, Rosmini F. Sibship size, birth order, and atopy in 11,371 Italian young men. J Allergy Clin Immunol. 1998;101:439–444. doi: 10.1016/s0091-6749(98)70350-1. [DOI] [PubMed] [Google Scholar]
- 15.Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy. 2002;32:43–50. doi: 10.1046/j.0022-0477.2001.01267.x. [DOI] [PubMed] [Google Scholar]
- 16.Lamartiniere CA Cotroneo MS, Fritz WA Wang J, Mentor-Marcel R Elgavish A. Genistein chemoprevention, timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132:552S–558S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- 17.Depue RH, Pike MC, Henderson BE. Estrogen exposure during gestation and risk of testicular cancer. J Natl Cancer Inst. 1983;71:1151–1155. [PubMed] [Google Scholar]
- 18.http://www.testdiet.com/5k96.htm
- 19.Doerge DR, Churchwell MI, Delclos KB. On-line sample preparation using restricted-access media in the analysis of the soy isoflavones, genistein and daidzein, in rat serum using liquid chromatography electrospray mass spectrometry. Rapid Commun Mass Spectrom. 2000;14:673–678. doi: 10.1002/(SICI)1097-0231(20000430)14:8<673::AID-RCM935>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 20.Bleavins MR, Iglesia FA, de la, McCay JA, White KL, Jr, Munson AE. Immunotoxicologic studies with CI-959, a novel benzothiophene cell activation inhibitor. Toxicology. 1995;98:111–123. doi: 10.1016/0300-483x(94)02985-4. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds CW, Herberman RB. In vitro augmentation of rat natural killer (NK) cell activity. J Immunol. 1981;126:1581–1585. [PubMed] [Google Scholar]
- 22.Franke AA, Custer LJ. Daidzein and genistein concentrations in human milk after soy consumption. Clin Chem. 1996;42:955–964. [PubMed] [Google Scholar]
- 23.Irvine CH, Fitzpatrick MG, Alexander SL. Phytoestrogens in soy-based infant foods: concentrations, daily intake, and possible biological effects. Proc Soc Exp Biol Med. 1998;217:247–253. doi: 10.3181/00379727-217-44229. [DOI] [PubMed] [Google Scholar]
- 24.Flynn KM, Ferguson SA, Delclos KB, Newbold RR. Effects of genistein exposure on sexually dimorphic behaviors in rats. Toxicol Sci. 2000;55:311–319. doi: 10.1093/toxsci/55.2.311. [DOI] [PubMed] [Google Scholar]
- 25.Day JK, Bauer AM, DesBordes C, Zhuang Y, Kim BE, Newton LG, Nehra V, Forsee KM, MacDonald RS, Besch-Williford C, Huang TH, Lubahn DB. Genistein alters methylation patterns in mice. J Nutr. 2002 Aug;132(8 Suppl):2419S–2423S. doi: 10.1093/jn/132.8.2419S. [DOI] [PubMed] [Google Scholar]
- 26.Lu Q, Wu A, Ray D, Deng C, Attwood J, Hanash S, Pipkin M, Lichtenheld M, Richardson B. DNA methylation and chromatin structure regulate T cell perforin gene expression. J Immunol. 2003 May 15;170(10):5124–32. doi: 10.4049/jimmunol.170.10.5124. [DOI] [PubMed] [Google Scholar]
- 27.Kawamata N, Inagaki N, Mizumura S, Sugimoto KJ, Sakajiri S, Ohyanagi-Hara M, Oshimi K. Methylation status analysis of cell cycle regulatory genes (p16INK4A, p15INK4B, p21Waf1/Cip1, p27Kip1 and p73) in natural killer cell disorders. Eur J Haematol. 2005 May;74(5):424–9. doi: 10.1111/j.1600-0609.2005.00417.x. [DOI] [PubMed] [Google Scholar]
- 28.Chapin RE, Harris MW, Davis BJ, Ward SM, Wilson RE, Mauney MA, Lockhart AC, Smialowicz RJ, Moser VC, Burka LT, Collins BJ. The effects of perinatal/juvenile methoxychlor exposure on adult rat nervous, immune, and reproductive system function. Fundam Appl Toxicol. 1997;40:138–57. doi: 10.1006/faat.1997.2381. [DOI] [PubMed] [Google Scholar]
- 29.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 30.Hori S, Carvalho TL, Demengeot J. CD4+CD25+ regulatory T cells suppress CD4+T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002;32:1282–1291. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 31.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+T cell regulate virus-specific primary and memory CD8+ T cell response. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu D, Liu H, Komai-Koma M, Campbell C, MaSharry C, Alexander J, Liew FY. CD4+CD25+ regulatory T cell suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J Immunol. 2003;170:394–399. doi: 10.4049/jimmunol.170.1.394. [DOI] [PubMed] [Google Scholar]
- 33.Oldenhove G, de Heusch M, Urbain-Vansanten G, Urbain J, Maliszewski C, Leo O, Moser M. CD4+CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J Exp Med. 2003;198:259–266. doi: 10.1084/jem.20030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curran EM, Berghaus LJ, Vernetti NJ, Saporita AJ, Lubahn DB, Estes DM. Natural killer cells express estrogen receptor-alpha and estrogen receptor-beta and can respond to estrogen via a non-estrogen receptor-alpha-mediated pathway. Cell Immunol. 2001;214:12–20. doi: 10.1006/cimm.2002.1886. [DOI] [PubMed] [Google Scholar]
- 35.Stimson WH. Oestrogen and human T lymphocytes: presence of specific receptors in the T-suppressor/cytotoxic subset. Scand J Immunol. 1988;28:345–350. doi: 10.1111/j.1365-3083.1988.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 36.Karpuzoglu-Sahin E, Zhi-Jun Y, Lengi A, Sriranganathan N, Ansar Ahmed S. Effects of long-term estrogen treatment on IFN-gamma, IL-2 and IL-4 gene expression and protein synthesis in spleen and thymus of normal C57BL/6 mice. Cytokine. 2001;14:208–17. doi: 10.1006/cyto.2001.0876. [DOI] [PubMed] [Google Scholar]
- 37.Karpuzoglu-Sahin E Hissong BD, Ansar Ahmed S. Interferon-gamma levels are upregulated by 17-beta-estradiol and diethylstilbestrol. J Reprod Immunol. 2001;52:113–127. doi: 10.1016/s0165-0378(01)00117-6. [DOI] [PubMed] [Google Scholar]
- 38.Maret A, Coudert JD, Garidou L, Foucras G, Gourdy P, Krust A, Dupont S, Chambon P, Druet P, Bayard F, Guery JC. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor alpha expression in hematopoietic cells. Eur J Immunol. 2003;33:512–521. doi: 10.1002/immu.200310027. [DOI] [PubMed] [Google Scholar]
- 39.Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol Pharmacol. 1998;54:105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- 40.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 41.Rasheed FN Bulmer JN, Dunn DT Menendez C, Jawla MF Jepson A, Jakobsen PH Greenwood BM. Suppressed peripheral and placental blood lymphoproliferative responses in first pregnancies: relevance to malaria. Am J Trop Med Hyg. 1993;48:154–160. doi: 10.4269/ajtmh.1993.48.154. [DOI] [PubMed] [Google Scholar]
- 42.Ohno S Nakajima Y, Inoue K Nakazawa H, Nakajin S. Genistein administration decreases serum corticosterone and testosterone levels in rats. Life Sci. 2003;74:733–742. doi: 10.1016/j.lfs.2003.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Korsgren M Persson CG, Sundler F Bjerke T, Hansson T Chambers BJ, Hong S Van Kaer L, Ljunggren HG Korsgren O. Natural killer cells determine development of allergen-induced eosinophilic airway inflammation in mice. J Exp Med. 1999;189:553–562. doi: 10.1084/jem.189.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohn L Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]


