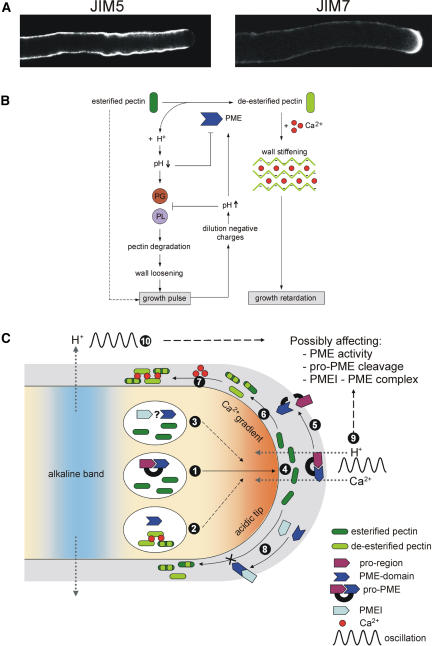
Figure 2.
Regulation of PME Activity in Pollen Tubes.
(A) Example of the distribution of relatively deesterified pectins and esterified pectins in N. tabacum pollen tubes by means of JIM5 and JIM7, respectively, immunolabeling. Notice the sudden transition from apical esterified pectin epitopes to deesterified pectin epitopes.
(B) Potential feedback mechanism for the regulation of apical PME activity in pollen tubes that could contribute to an oscillatory growth pattern. PG, polygalacturonase; PL, pectate lyase.
(C) Regulation of PME activity by its pro-region, PMEIs, and oscillating ion fluxes. (1) The pro-region functions as an intramolecular inhibitor of PME activity, preventing the premature deesterification of pectins prior to secretion. (2) Expression of only the PME domain can lead to premature, intravesicular, demethylesterification of pectins, causing inhibition of tube growth. (3) It remains to be seen if PMEIs can inhibit premature deesterification of PME isoforms lacking a pro-region. (4) Pectins are secreted in a highly methylesterified state. (5) Removal of the pro-region by cleavage of pro-PMEs is necessary for the PME domain to become enzymatically active. (6) The active PME domain catalyzes the demethylesterification of pectins. (7) Ca2+ binds cooperatively to the free carboxyl groups, which causes rigidification of the cell wall. (8) PMEIs are able to inhibit the PME activity in the cell wall by forming a 1:1 reversible complex. (9) The oscillatory influx of H+ and Ca2+ ions, which maintain the apical acidic domain and the tip-focused Cai2+ gradient, respectively, through the apical cell wall might directly influence the PME activity, the conditions necessary for cleavage of pro-PME, and the formation of the pH-dependent PMEI-PME complex. (10) The same is true for the oscillatory efflux of H+ ions maintaining the alkaline band at the base of the clear zone.