Figure 1.
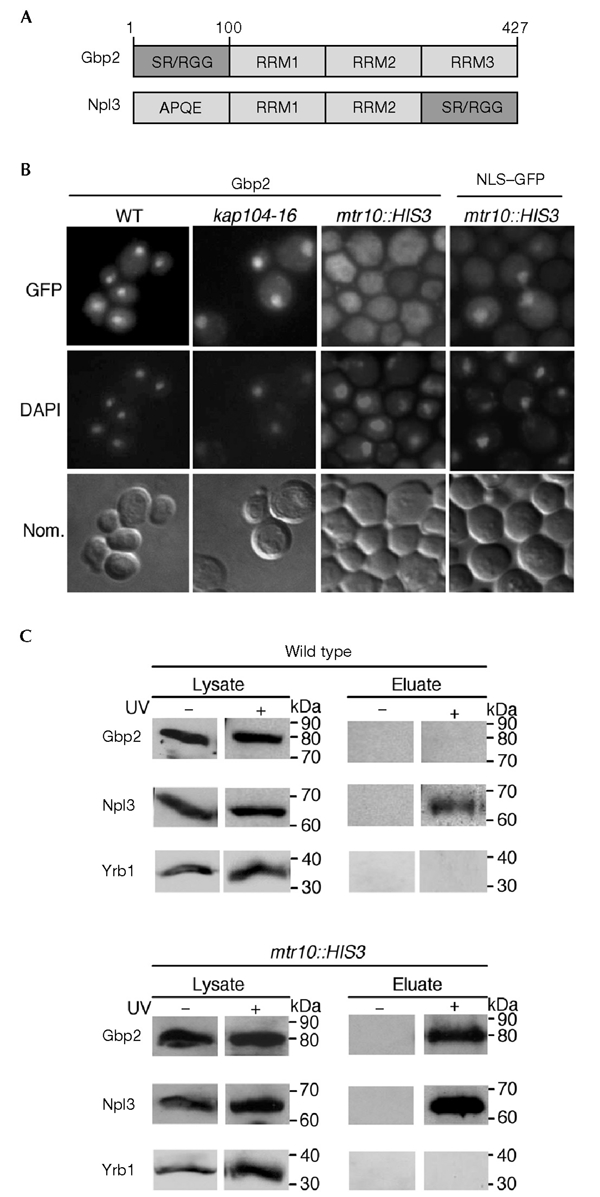
Gbp2 is a messenger RNA-binding protein that is transported into the nucleus by Mtr10. (A) Domain organization of Gbp2 and Npl3. Gbp2 consists of an amino-terminal domain with SR/RGG repeats followed by three RNA recognition motifs (RRMs). Npl3 contains two RRMs, a carboxy-terminal SR/RGG domain and an N-terminal domain with repeats of the amino-acid residues APQE. (B) Gbp2 is localized to the nucleus at steady state and accumulates in the cytoplasm of mtr10::HIS3 cells. Subcellular localization of Gbp2–GFP (green fluorescent protein) is shown in a wild-type strain (WT), in kap104-16 and in mtr10::HIS3 strains, shifted to 37 °C for 30 min. As a control, the localization of a nuclear localization signal–GFP reporter is shown under the same conditions in mtr10::HIS3 cells. The DNA was stained with DAPI (4,6-diamidino-2-phenylindole), and the cells were photographed using Nomarski optics (Nom.). (C) Ultraviolet (UV) crosslinking of Gbp2 to poly(A)+ RNA. Wild-type cells and mtr10::HIS3 cells that express Gbp2–GFP were grown to log phase and exposed to UV light for crosslinking (+ or − UV irradiation). The lysates were taken before the material was applied to an oligo(dT)-cellulose column. The eluates containing the crosslinked poly(A)+ RNA and bound proteins were treated with RNases. Proteins were analysed by SDS–polyacrylamide gel electrophoresis, followed by western blotting. The immunoblot was probed with anti-GFP antibodies and with anti-Npl3 antibodies. As a control for non-specific binding, anti-Yrb1 antibodies were used in a similar experiment.
