Figure 2.
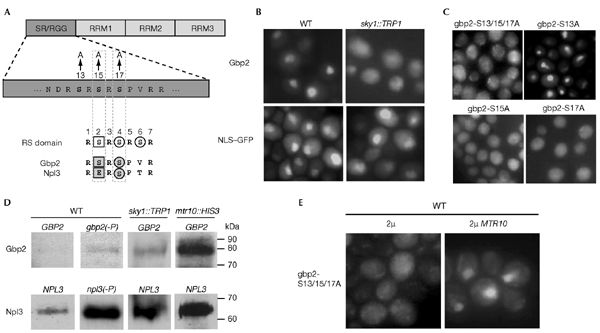
Sky1 is important for the nuclear localization of Gbp2 and for the efficiency of RNA release from Gbp2. (A) Domain organization of Gbp2 and indication of the mutations generated by site-directed mutagenesis. Bottom, an alignment of the phosphorylation sequence (RS domain) of SR-specific protein kinases (SRPKs) in mammals and the Sky1 phosphorylation recognition sequence in Npl3 and Gbp2 is shown. The white square indicates the position of an initial phosphorylation site that has been suggested to prime the activity of SRPKs, white circles indicate the phosphorylation sites for SRPKs. Shaded squares and circles indicate the corresponding residues in the yeast shuttling SR proteins. (B) Localization of Gbp2–GFP (green fluorescent protein) and NLS–GFP in logarithmic growing wild-type (WT) and sky1::TRP1 cells was detected by fluorescence microscopy. (C) Localization of the Gbp2 mutants in vivo. Wild-type strains that express mutant gbp2–GFP were grown to log phase and subcellular localization was determined by fluorescence microscopy. (D) Ultraviolet-crosslinking experiments were carried out in: a wild-type strain that expresses wild-type GBP2; a wild-type strain either expressing mutant GBP2 (gbp2-S13/15/17A, designated as gbp2(−P)) or mutant Npl3 (npl3-27, designated as npl3(−P)); sky1::TRP1 cells; and mtr10::HIS3 cells that express wild-type GBP2. All GBP2 constructs were expressed as GFP fusion proteins. Eluates were loaded in identical amounts in each lane. The western blot was probed with anti-GFP antibodies (top) and anti-Npl3 antibodies (bottom). (E) Wild-type strains that bear gbp2-S13/15/17A-GFP on a CEN plasmid and either MTR10 or no additional gene on a 2μ plasmid were grown to log phase and the subcellular localization of Gbp2 was determined by fluorescence microscopy. RRM, RNA recognition motif.
