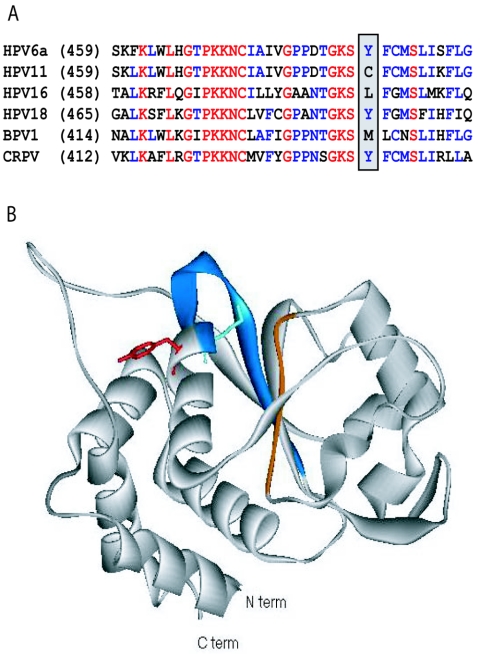
FIG. 5.
Structure of HPV18 showing the possible binding region of biphenylsulfonacetic acid inhibitors. (A) Sequence alignment of part of the helicase domains of the E1 proteins from two low-risk (HPV6 and -11) and two high-risk (HPV16, and -18) HPV types as well as those of bovine papillomavirus 1 (BPV1) and CRPV. Fully conserved residues are red, highly conserved residues are blue, and others are black. The positions of Tyr-486 in HPV6a E1 and of the corresponding amino acids in other E1 proteins are highlighted. For the entire helicase domain, the two low-risk sequences are 93% identical, whereas the two high-risk sequences are 65% identical. For other possible pair-wise combinations, the sequence identity ranges from 50% to 60%. (B) Ribbon diagram for the HPV18 E1 helicase domain (1). The Walker A and B motifs involved in ATP binding are blue and orange, respectively. The side chains of the catalytic Lys-490 and Tyr-492 are shown extending in opposite directions from the α-helix (equivalent to Lys-484 and Tyr-486, respectively, in HPV6 E1). This illustration was created using Viewer Lite version 5.0 (Accelrys).