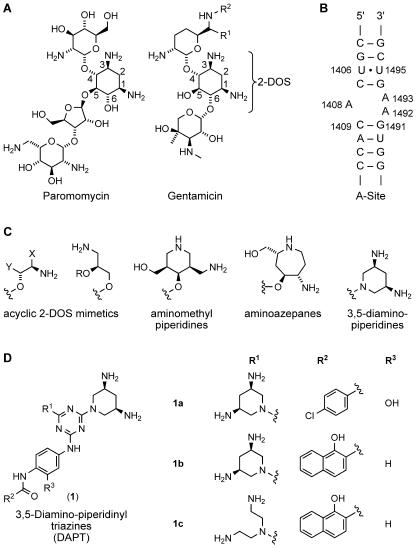
FIG. 1.
(A) Natural aminoglycoside antibiotics that target the bacterial ribosomal decoding site. The natural products are derived from 2-DOS by glycosidic substitutions at positions 4 and 5 (paromomycin) or 4 and 6 (gentamicin). Gentamicin is a mixture of gentamicin C1 (R1 = R2 = CH3), gentamicin C1A (R1 = R2 = H), and gentamicin C2 (R1 = CH3, R2 = H). (B) Secondary structure of the bacterial decoding site (A-site) within 16S rRNA. Residues that are involved in aminoglycoside interactions are labeled. (C) Scaffolds that were designed and studied as structural mimetics of the aminoglycoside 2-DOS core (2, 22, 28). 3,5-Diaminopiperidines are described in this report. (D) Structure of DAPT antibacterials (compounds designated by the number 1). Compounds 1a to 1c contain at least one cis-3,5-diamino-piperidinyl group.