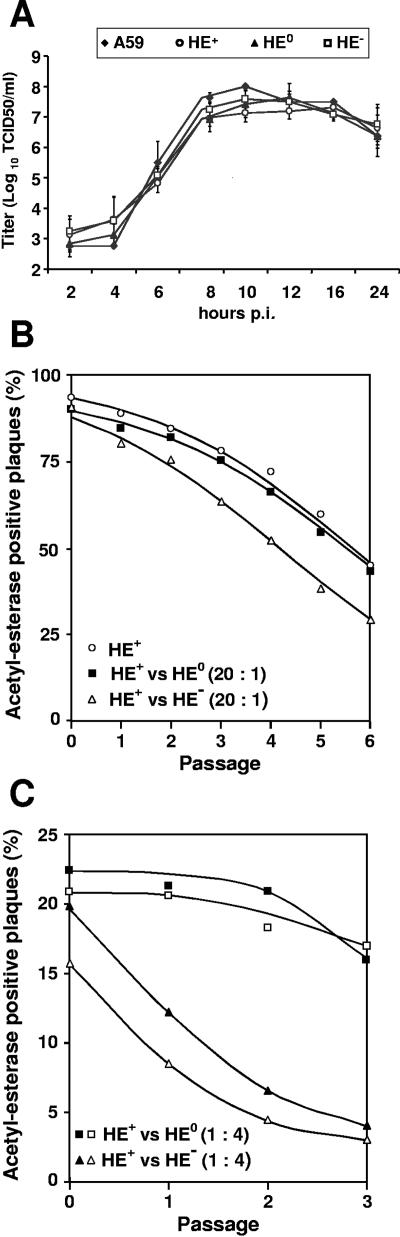
FIG. 5.
In vitro growth properties of rMHV-HE+, -HE0, and -HE−. (A) Single-step growth kinetics of MHV recombinants. LR7 cells were infected with MHV-A59 or with each of the recombinant MHVs at an MOI of 10. Viral infectivity present in the culture medium at different times postinfection was determined by titration in LR7 cells by end-point dilution, and titers (50% tissue culture infective doses/ml [TCID50/ml]) were calculated. (B and C) Relative fitness of recombinant MHVs, as measured in mixed propagation-competition assays. rMHV-HE+ was serially passaged in LR7 cells either alone or in combination with rMHV-HE0 or rMHV-HE−, mixed at the indicated ratios. The propagation-competition experiments shown were performed with three different sets of independently isolated rMHVs (indicated by open and solid squares and triangles). Monolayers were inoculated at an MOI of 0.01. Tissue culture supernatants were harvested at 16 h p.i. and analyzed by plaque assays and in situ esterase staining. The graphs show the percentage of acetylesterase-positive plaques (y axis) in each of the passages (x axis). For graph B, at least 200 plaques were counted for each sample after every passage (average, 440 ± 170), and for graph C, at least 700 plaques were counted for each sample (average, 990 ± 340).