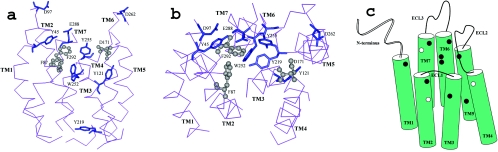
FIG. 5.
Distinct functional sites for SDF-1α and SMM chemokines highlighted on a hypothetical structural model of CXCR4. As detailed in Table 4, the residues involved in both SDF-1α and SMM chemokine binding are highlighted in the lighter color and represented in the ball-and-stick format, whereas those selectively involved in SMM chemokine binding are highlighted in the darker color. Only the TM domains, with side (a) and top (b) views, are shown for simplicity. The model was built based on the previously published structural model of CXCR4 by our laboratory (19). (c) Schematic illustration of the locations of residues important for ligand binding on CXCR4 TM and extracellular domains. The residues involved in the binding activities of both SDF-1α and SMM chemokines are highlighted with white spots, whereas those selectively involved in SMM chemokine binding (most of which overlap with HIV-1 binding) are highlighted with black spots. Such overlapping sites between HIV-1 and SMM chemokines may serve as a potential target recognized by new selective anti-HIV inhibitors.