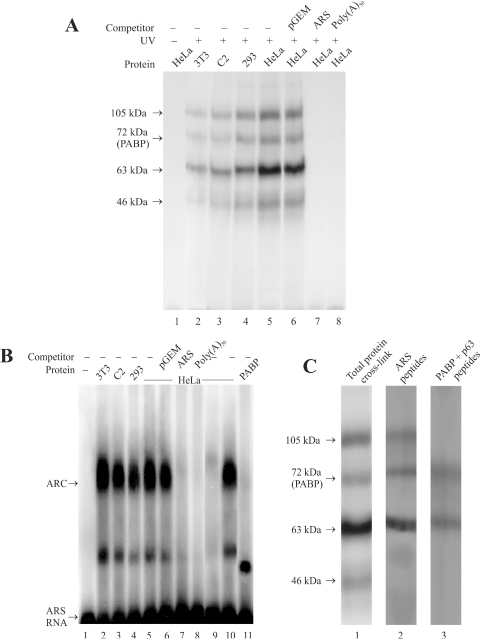
Figure 1.
Formation of ARS RNA–protein complex. (A) RNA–protein crosslinking by UV. The in vitro synthesized [32P]-labeled ARS RNA (≈3 ng, 3 × 105 c.p.m.) was incubated with different cell extract (≈60 µg protein) in the flat cap of a 0.2 ml PCR tube. Following the UV treatment, the samples were treated with RNaseA/RNase T1, analyzed by 10% SDS–PAGE and autoradiographed as described under experimental procedures. Cell extracts from mouse NIH3T3 fibroblasts (lane 2) and C2 myoblasts (lane 3), and human HEK293 (lane 4) and HeLa cells (lane 5) were used for these studies. One sample containing HeLa cell extract (lane 1) was analyzed without UV treatment as a control. Approximately 300 ng of the non-radioactive pGEM-T (lane 6), ARS (lane 7) or poly(A)50 RNA (lane 8) was used for competition studies. (B) Analysis of RNP complex by REMSA. The in vitro synthesized [32P]-labeled ARS RNA (≈1 ng, 1 × 105 c.p.m.) was incubated with different cell extracts (20 µg) as indicated above each lane. The unbound RNA was digested with RNaseT1 and subjected to electrophoresis in a 2% agarose as described in the experimental procedures. Lane 1, radioactive ARS RNA incubated without the cell extract; lanes 2–5, radioactive ARS RNA incubated with NIH3T3, C2, HEK293 and HeLa cell extracts, respectively. Lanes 6–8, competition with 100 ng of non-radioactive pGEM-T, ARS, or poly(A)50 RNA, respectively. Lane 9, cell extract was pre-incubated with the PABP antibody and precleared with protein A-sepharose beads before being used for REMSA. Approximately 20 µg of PABP deficient cell extract was used for REMSA. Lane 10, cell extract was similarly treated with the GFP antibody (BD Biosciences) before being used for REMSA. Lane 11, radiolabeled ARS RNA (≈ 1 ng, 1 × 105 c.p.m.) incubated with ≈2 ng of purified 6×His-PABP. (C) Analysis of RNP complexes by SDS–PAGE. RNP complex formation was initiated as described above and the samples were irradiated by UV before being resolved in a 2% agarose gel. The RNP bands (as shown in Figure 1B) were excised from the gel, treated with RNaseA/RNase T1, and analyzed by 10% SDS–PAGE as described in the Materials and Methods. Lane 1, ARS RNA and HeLa extracts treated with UV and analyzed before gel purification. Lane 2, polypeptides from the gel purified slower migrating ARC. Lane 3, polypeptides from the faster migrating minor complex.