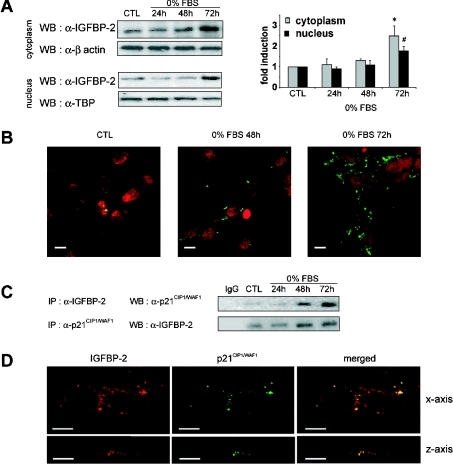
Figure 3. Interaction between intracellular IGFBP-2 and p21CIP1/WAF1 during growth inhibition.
Sub-confluent MLE-12 cells were cultured in HITES medium, in the presence of serum for 24 h (CTL) or in the absence of serum (0% FBS) for 24, 48 or 72 h. (A) Western blot (WB) analysis of cytoplasmic and nuclear IGFBP-2 protein. Equal amounts of cytoplasmic and crude nuclear protein extracts from control cells (CTL) and from serum-deprived cells were analysed by Western blotting with anti-IGFBP-2 antibody. Crude nuclear extracts consisted of cell nuclei that were purified by sucrose density centrifugation, as described in Experimental section. Antibodies against β-actin and TATA-binding protein (α-TBP) were used as controls for protein loading in the cytoplasmic and nuclear extracts respectively. The histogram shows a quantitative representation of cytoplasmic and nuclear levels of IGFBP-2 protein obtained from laser densitometric analysis of three independent experiments. Densitometry results were expressed as fold induction. * and #, P<0.05 compared with control. (B) Subcellular localization of IGFBP-2 in proliferating and growth-inhibited MLE-12 cells. Fixed and permeabilized cells were immunostained with primary antibody to IGFBP-2 and secondary FITC-conjugated anti-rabbit antibody. Immunofluorescence staining of IGFBP-2 and nuclei stained with propidium iodide were visualized by confocal microscopy. Scale bars represent 10 μm. (C) Coimmunoprecipitation analysis of the IGFBP-2–p21CIP1/WAF1 interaction. Total protein extracts from cultured MLE-12 cells were immunoprecipitated (IP) with anti-IGFBP-2 antibody and p21CIP1/WAF1 was detected with anti-p21CIP1/WAF1 antibody in the precipitated complex on a Western blot as described in the Experimental section (upper panel). Similarly, p21CIP1/WAF1 was immunoprecipiated with anti-p21CIP1/WAF1 antibody and Western blotted with anti-IGFBP-2. Representative blots from three independent experiments are shown. (D) Subcellular colocalization of IGFBP-2 and p21CIP1/WAF1 in growth-inhibited MLE-12 cells. MLE-12 cells were grown for 72 h in the absence of serum. Images of fixed and permeabilized cells were captured by confocal microscopy with respect to both x- (upper panels) and z-axes (lower panels). Cells were immunostained with anti-IGFBP-2 antibody followed by rhodamine–anti-(goat IgG) secondary antibody (red in left panels). Immunostaining of p21CIP1/WAF1 was carried out using anti-p21CIP1/WAF1 antibody which had been labelled with Alexa Fluor™ 555 fluorescent dye (green in middle panels). Colocalization of IGFBP-2 and p21CIP1/WAF1 was visualized by merging both staining patterns (right panels). Scale bars represent 10 μm.