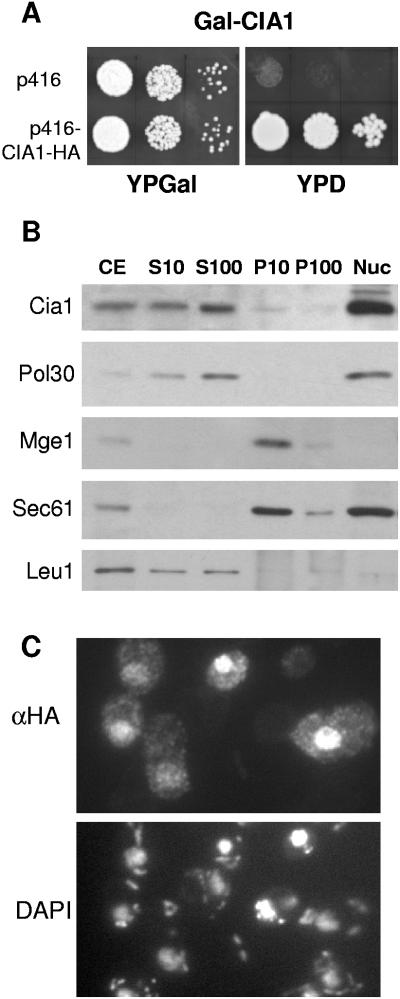
FIG. 2.
The essential Cia1 is a soluble protein in the nucleus and cytosol. (A) Growth of Cia1-depleted cells. Gal-CIA1 cells carrying a galactose-regulatable (Gal) CIA1 gene were transformed with vector p416 without or with a CIA1-HA fusion gene. Cells were grown for 2 × 2 days at 30°C on agar plates containing rich media supplemented with galactose (YPGal) or glucose (YPD). Tenfold serial dilutions are shown. (B) Subcellular fractionation and immunoblot analysis. Wild-type cells were disrupted by removal of the cell wall and homogenization with a Douncer in 0.6 M sorbitol, 20 mM HEPES-KOH, pH 7.4, 1 mM dithiothreitol, and protease inhibitors, followed by differential centrifugation. After discarding a low-speed pellet containing unbroken cells, nuclei, and debris, the cell extract (CE) was centrifuged at 10,000 × g for 10 min to separate soluble proteins (S10) from a fraction enriched in organelles (P10). The S10 fraction was further centrifuged at 100,000 × g for 30 min to remove membranes (P100) from soluble proteins (S100). Nuclei (Nuc) were purified separately using a Ficoll density gradient (1). Equal amounts of protein (20 μg/per lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted, and immunostained for Cia1 or marker proteins of the nucleus (DNA polymerase-associated Pol30), mitochondria (Mge1), endoplasmic reticulum (translocon subunit Sec61), and the cytosol (Leu1). (C) In situ localization of Cia1. Wild-type cells were transformed with the high-copy-number expression vector p426 containing CIA1 fused to the HA tag sequence. Log-phase cells were fixed with 2.4% (wt/vol) formaldehyde, permeabilized, and labeled with monoclonal anti-HA (α-HA) antibodies, followed by fluorophore-conjugated secondary antibodies. DNA was counterstained with DAPI (4′,6′-diamidino-2-phenylindole) to show the positions of the nucleus and mitochondria.