Abstract
In L6 myoblasts, insulin receptors with deletion of the C-terminal 43 amino acids (IRΔ43) exhibited normal autophosphorylation and IRS-1/2 tyrosine phosphorylation. The L6 cells expressing IRΔ43 (L6IRΔ43) also showed no insulin effect on glucose uptake and glycogen synthase, accompanied by a >80% decrease in insulin induction of 3-phosphoinositide-dependent protein kinase 1 (PDK-1) activity and tyrosine phosphorylation and of protein kinase B (PKB) phosphorylation at Thr308. Insulin induced the phosphatidylinositol 3 kinase-dependent coprecipitation of PDK-1 with wild-type IR (IRWT), but not IRΔ43. Based on overlay blotting, PDK-1 directly bound IRWT, but not IRΔ43. Insulin-activated IRWT, and not IRΔ43, phosphorylated PDK-1 at tyrosines 9, 373, and 376. The IR C-terminal 43-amino-acid peptide (C-terminal peptide) inhibited in vitro PDK-1 tyrosine phosphorylation by the IR. Tyr→Phe substitution prevented this inhibitory action. In the L6hIR cells, the C-terminal peptide coprecipitated with PDK-1 in an insulin-stimulated fashion. This peptide simultaneously impaired the insulin effect on PDK-1 coprecipitation with IRWT, on PDK-1 tyrosine phosphorylation, on PKB phosphorylation at Thr308, and on glucose uptake. Upon insulin exposure, PDK-1 membrane persistence was significantly reduced in L6IRΔ43 compared to control cells. In L6 cells expressing IRWT, the C-terminal peptide also impaired insulin-dependent PDK-1 membrane persistence. Thus, PDK-1 directly binds to the insulin receptor, followed by PDK-1 activation and insulin metabolic effects.
The interaction of insulin with its cell surface receptors leads to the activation of phosphatidylinositol 3-kinase (PI 3-kinase, or PI 3-K) and generation of phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3] at the inner surface of the cell membrane (19). PtdIns(3,4,5)P3 generation leads to the activation of a group of AGC family protein kinases, including different protein kinase B (PKB)/Akt isoforms, p70 ribosomal S6 kinase, serum- and glucocorticoid-induced protein kinase, and atypical protein kinase C (1, 9, 11, 12, 24, 28, 33). These kinases play key roles in the regulation of cell metabolism, proliferation, and survival by insulin, as well as other growth factors (4). How the AGC kinases are activated following insulin stimulation of PI 3-kinase has been extensively investigated (41). PKB/Akt activation requires phosphorylation at two highly conserved Ser/Thr residues by 3-phosphoinositide-dependent protein kinase 1 (PDK-1) (22) and by a distinct 3-phosphoinositide-dependent protein kinase 2 (PDK-2), recently identified as the mammalian target of rapamycin (21, 10, 36). Thus, PDKs represent master regulators of AGC kinase signal transduction (38, 40, 44). PDK-1 is composed of a C-terminal PH domain and a catalytic domain similar to the catalytic domains of PKA, PKB, and PKC(3). After stimulation by insulin, PI 3-kinase-generated lipids bind the PH domain of PDK-1, promoting its translocation to the membrane, together with that of other PH domain-containing proteins, such as PKB/Akt (27). In the membrane, PDK-1 achieves the appropriate conformation and reaches the appropriate compartment for optimal activation (39). Previous reports showed that phosphorylation of Ser241 in the activation loop is necessary for PDK-1 activity (8). There is also evidence that PDK-1 undergoes tyrosine phosphorylation in response to several growth factors (31). Studies with the tyrosine phosphatase inhibitor pervanadate indicate that full activation of PDK-1 requires phosphorylation at Tyr373/376 in 293 cells overexpressing insulin receptors (IRs) (30). It has also been shown that both sites can be phosphorylated by v-Src tyrosine kinase in vitro, and coexpression of v-Src leads to tyrosine phosphorylation and activation of PDK-1 in 293 cells (20, 30). Since Src kinases have been implicated in insulin signaling, it has been suggested that in the 293 cells, insulin regulation of PDK-1 activity is accomplished by a Src family kinase (20). Whether other insulin-dependent tyrosine kinases are also involved is still unclear, however. How the interaction with the membrane affects PDK-1 function also needs to be further defined.
In the present work, we have analyzed the mechanism of PDK-1 activation by insulin in L6 skeletal-muscle cells expressing a C-terminally truncated insulin receptor. We show that in response to insulin, PDK-1 directly binds to the insulin receptor C terminus and is tyrosine phosphorylated by the insulin receptor kinase. These events are necessary for activation of glucose uptake and glycogen synthesis by insulin.
MATERIALS AND METHODS
Materials.
Media, sera, and antibiotics for cell culture and the Lipofectamine reagent were from Invitrogen (Invitrogen Corporation, Paisley, United Kingdom). Antibodies against PDK-1, insulin receptor α (no. 06-544) and β (no. 06-492) subunits, IRS-1, IRS-2, phospho-Thr308 Akt1/PKBα, and phosphotyrosine were purchased from Upstate Biotechnology (Lake Placid, NY). Akt1/2 and ERK1 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-Ser473 Akt was obtained from Cell Signaling Technology (Beverly, MA). Protein A-Sepharose beads were purchased from Pierce (Rockford, IL), sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) reagents from Bio-Rad (Richmond, VA), and radiochemicals and Western blotting and enhanced chemiluminescence (ECL) reagents from Amersham Biosciences (Arlington Heights, IL). All other chemicals were from Sigma (St. Louis, MO). The pco11 expression vector containing the cDNA of the truncated human insulin receptor and the pcDNA3 expression vector containing the FLAG epitope were generously donated by D. Accili (Columbia University) and A. Leonardi (Federico II University of Naples, Naples, Italy). The pCEFL vector encoding the myristilated p110 PI 3-K subunit was generously donated by M. Chiariello (IEOS, CNR, Naples, Italy). Amino acids, resins, and reagents for peptide synthesis and purification were from Novabiochem (Laufelfingen, Switzerland) and Sigma-Aldrich (St. Louis, MO).
Cell culture and transfection and cloning of the FLAG43 peptide.
The L6 cell clone expressing 3.2 × 103 wild-type human insulin receptors (L6hIR) has been previously characterized and reported (6). The cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 100 IU/ml penicillin, 100 IU/ml streptomycin, and 2% l-glutamine in a humidified CO2 incubator, as described by Caruso et al. (6).
The pco11 expression vector containing the cDNA of the truncated human insulin receptor was stably transfected in L6 parental myoblasts using the Lipofectamine method according to the manufacturer's instructions. Individual G418-resistant clones were selected by the limiting-dilution technique (G418 effective dose, 0.8 mg/ml). The expression of the truncated human insulin receptor by the individual clones was quantitated by equilibrium 125I-insulin binding as described previously (17) and confirmed by Western blotting with insulin receptor α-subunit antibodies.
The cDNA encoding amino acids 1340 to 1382 of the insulin receptor COOH terminus was obtained by PCR amplification using the oligonucleotides 5′-GAAGGATCCGCGGGGGGCCGGGAT-3′and 5′-CCGCTTAAGTTAGGAAGGATTGGA-3′and introducing BamHI and EcoRI sites, respectively, at the 5′ and the 3′ end sites. The PCR product was digested with BamHI and EcoRI and cloned into the BamHI and EcoRI sites of the pcDNA3-FLAG expression vector containing the Ampr selectable marker (FLAG43 cDNA). Transient transfection of the construct was performed the by the Lipofectamine method according to the manufacturer's instructions.
Western blot analysis and immunoprecipitation and overlay blot procedures.
For Western blot analysis and immunoprecipitation and overlay blot procedures, cells were solubilized in lysis buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 10 mM EDTA, 10 mM Na2P2O7, 2 mM Na3VO4, 100 mM NaF, 10% glycerol, 1% Triton X-100, 1 mM phenylmethylsulfonylfluoride, 10 μg/ml aprotinin) for 2 h at 4°C. Cell lysates were clarified by centrifugation at 5,000 × g for 20 min, separated by SDS-PAGE, and transferred onto 0.45-μm Immobilon-P membranes (Millipore, Bedford, MA). Upon incubation with primary and secondary antibodies, immmunoreactive bands were detected by ECL according to the manufacturer's instructions. Cell lysate immunoprecipitations were accomplished as previously described (16).
Insulin receptors were partially purified by wheat germ agglutinin (WGA) affinity chromatography, and the insulin receptor number in the eluates was determined as described previously (18). Overlay blotting with biotinylated PDK-1 was accomplished as reported previously (8). Filters were revealed by ECL and autoradiography.
PI 3-kinase and PDK-1 activities and PDK-1 phosphorylation.
To assay PI 3-kinase activity, cells were stimulated with 100 nM insulin at 37°C for 5 and 30 min as indicated. The cells were then solubilized for 40 min at 4°C in 50 mM HEPES, pH 7.5, 150 mM NaCl, 10% glycerol, 1% NP-40, 10 mM EDTA, 10 mM Na4P2O7, 1 mM Na3VO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 100 mM NaF, 1 mM phenylmethylsulfonyl fluoride (TAN buffer). Aliquots of the lysates were precipitated with phosphotyrosine antibodies coupled to protein A-Sepharose for 2 h at 4°C. PI 3-kinase activity was determined in the immunoprecipitates by measuring PIP3 production as described previously (15).
To assay PDK-1 activity, cells were solubilized in 50 mM Tris, pH 7.5, 0.1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 50 mM NaF, 10 mM sodium glycerophosphate, 5 mM Na4P2O7, 1 mM Na3VO4, 0.1% 2-mercaptoethanol, 1 μM microcystin LR, 0.2 mM phenylmethylsulfonyl fluoride, 25 μg/ml aprotinin (extraction buffer). Cell lysates were clarified by centrifugation at 5,000 × g for 20 min and immunoprecipitated with PDK-1 antibodies. PDK-1 activity was then assayed in the immunoprecipitates by measuring phosphorylation of the PDKtide substrate using the Upstate Biotechnology kit (Charlottesville, VA; kit no. 14-280) according to the manufacturer's instructions. PDK-1 catalytic competence was further determined by assaying autophosphorylation at Ser241. In these measurements, cells were solubilized and Western blotted with specific pospho-Ser241-PDK-1 antibodies as outlined above.
To analyze the phosphorylation of PDK-1 in vitro, the cells were solubilized with TAT buffer (42) and precipitated with PDK-1 antibodies. The precipitated PDK-1 was incubated with protein A-Sepharose for 2 h at 4°C and further incubated with insulin receptors (250 fmol/assay). Phosphorylation reactions were initiated by adding 2 mM CTP, 2 μM ATP, 10 mM HEPES, pH 7.4, 0.02% Triton X-100, 5 mM MnCl2, 7 mM MgCl2 (final concentrations) and prolonged for 30 min at 22°C. The phosphorylated proteins were separated by SDS-PAGE and analyzed by Western blotting with phosphotyrosine antibodies.
Effects of in vivo insulin treatment on PDK-1 signaling.
For the experiments, C57/BL6 mice fasted overnight and then were injected intraperitoneally with insulin (0.15 U/g body weight) or an equal volume of saline. After 5 min, the animals were sacrificed by decapitation, and gastrocnemius muscles and epidydimal fat pads were quickly dissected and frozen in liquid nitrogen. The tissues were weighed, finely pulverized, and stored at −80°C as described previously (42). These preparations were used for studying PDK-1 coprecipitation with insulin receptors as described above.
Thymidine incorporation, 2-deoxy-d-glucose uptake, and glycogen synthase activity.
Thymidine incorporation was determined as previously reported (18). Briefly, L6hIR and L6Δ43 cells were seeded in six-well plates and, 18 h later, incubated in Dulbecco's modified Eagle's medium supplemented with 0.25% bovine serum albumin. The cells were subsequently maintained for 16 h in the absence or the presence of 100 nM insulin, followed by the addition of 500 nCi/ml [3H]thymidine. After four more hours, the cells were rinsed with ice-cold 0.9% NaCl, precipitated with 20% trichloroacetic acid, and solubilized with 1 N NaOH. The radioactivity incorporated into the nuclei was finally quantitated by liquid scintillation counting.
For determining 2-deoxyglucose (2-DG) uptake, cells were maintained in glucose-free buffer (25 mM HEPES, pH 7.4, 125 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, 1 mM CaCl2, 0.25% bovine serum albumin) for 3 h, followed by stimulation with 100 nM insulin for 30 min and incubation in glucose-free buffer containing 2-DG (final concentration, 0.15 mM) and 0.5 μCi/assay [14C]2-DG for ten more min (5). The cells were solubilized, and 2-DG uptake was determined by liquid scintillation counting.
Glycogen synthase activity was determined as previously described (5). Briefly, L6hIR and L6Δ43 myotubes were incubated in HEPES buffer for 3 h, stimulated with 100 nM insulin, resuspended in 10 mM EDTA, and then sonicated for 10 s at 300 W. After centrifugation for 10 min at 2,000 × g, the supernatants were added to a reaction mixture containing 40 mM Tris-HCl, pH 7.8, 25 mM NaF, 20 mM EDTA, 10 mg/ml glycogen, 7.2 mM UDP-glucose, and 0.05 mCi [14C]UDP-glucose in the absence or the presence of 6.7 mM glucose 6-phosphate at 30°C. Twenty minutes later, the reaction was terminated by spotting the mixture on P81 phosphocellulose filters and precipitation with ice-cold ethanol. Filter-precipitated radioactivity was quantitated by liquid scintillation.
Purified plasma membrane preparations.
Purified plasma membrane preparations were obtained as previously reported (37, 32), with slight modifications as in Caruso et al. (7). Briefly, the cells were washed in ice-cold phosphate-buffered saline and homogenized in 500 μl of ice-cold fractionation buffer (20 mM HEPES-NaOH, pH 7.4, 250 mM sucrose, 25 mM sodium fluoride, 1 mM sodium pyrophosphate, 0.1 mM sodium orthovanadate, 2 μM microcystin LR, 1 mM benzamidine) by passing them 10 times through a 22-gauge needle. Subcellular fractionation was performed by differential centrifugation as described previously (32, 37). This fractionation procedure, which separates membrane fractions on the basis of differential centrifugation, generates four membrane fractions. Based on Western blotting, the cell surface markers transferrin receptor, 5′-nucleotidase, and Na-K/ATPase selectively localized to the plasma membrane fraction.
Peptide synthesis.
The 43-residue C-terminal domain of IR (fragment 1340 to 1382) and the Y→F-mutated peptide were prepared in the N-terminal acetylated form by solid-phase peptide synthesis, following standard 9-fluorenylmethoxy carbonyl (Fmoc) protocols (13), and purified to homogeneity by reverse-phase high-performance liquid chromatography. Peptide purity and identity were assessed by liquid chromatography-mass spectrometry analysis. The lyophilized peptide was used in the assays without further manipulation after dissolution in the indicated buffers.
RESULTS
Insulin action in L6 cells expressing hIRΔ43.
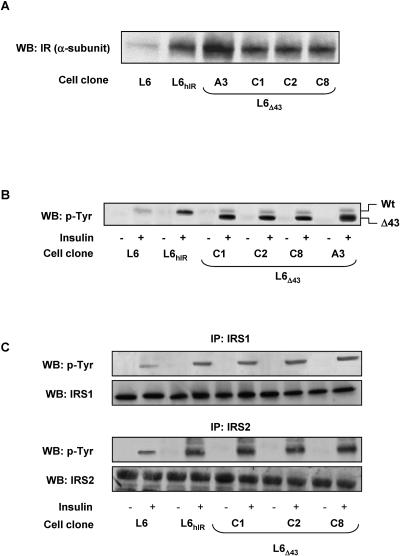
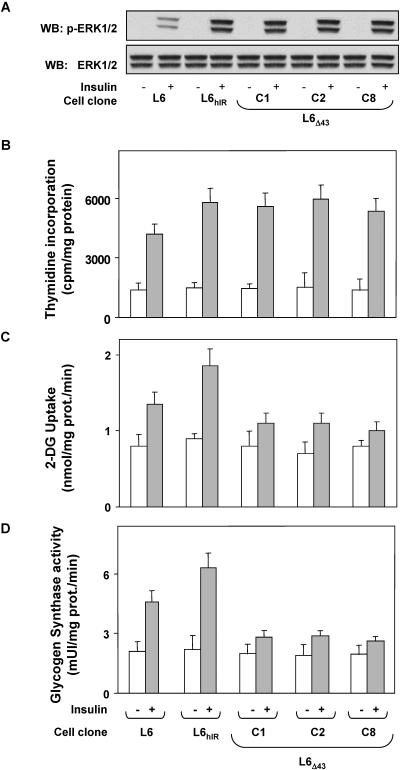
We stably transfected the human insulin receptor cDNA featuring a 43-amino-acid deletion at the C terminus (hIRΔ43) in the L6 skeletal-muscle cells. Several individual clones of L6 cells expressing the hIRΔ43 were selected, and four of these clones (L6Δ43 cells; clones C1, C2, C8, and A3) were studied in detail. Compared to the parental L6 cells, these clones overexpressed the hIRΔ43 by 8- (C1, C2, and C8) and 12-fold (A3), respectively, above the expression level of endogenous insulin receptors (Fig. 1A). L6 cells overexpressing wild-type human insulin receptors by eightfold (L6hIR) were also analyzed. Insulin-dependent autophosphorylation closely paralleled the insulin receptor levels in these clones and in parental L6 cells, as well as in L6hIR cells (Fig. 1B). Insulin-dependent phosphorylation of IRS-1 and IRS-2 paralleled insulin receptor levels in all of these cells, indicating that the hIRΔ43 features preserved autophosphorylation and phosphorylation of the major substrates in the L6 cells (Fig. 1C). The activation of these early steps of the insulin signaling cascade was accompanied by increased phosphorylation of ERK1/2 and incorporation of [3H]thymidine by 4-fold in both the hIRΔ43-expressing cells and the L6hIR cells and by 2.5-fold in the parental untransfected L6 cells (Fig. 2A and B). However, insulin activation of 2-deoxy-d-glucose uptake and glycogen synthase in the L6Δ43 cells were reduced by two- and fourfold compared to the L6 and the L6hIR cells, respectively (Fig. 2C and D).
FIG. 1.
Expression of hIRΔ43 in L6 skeletal-muscle cells. (A) Untransfected L6 myoblasts (L6) and myoblasts expressing 3 × 103 wild-type (Wt) (L6hIR) (6) or carboxy-terminally truncated insulin receptors (L6Δ43; clone A3, 4 × 103 insulin receptors/cell; clones C1, C2, and C8, 3.1 × 103 insulin receptors/cell) were solubilized, and equal amounts of proteins (50 μg/sample) were analyzed by Western blotting (WB) using IR α-subunit antibodies as described in Materials and Methods. The blots were revealed by ECL and autoradiography. (B) The cells were stimulated with 100 nM insulin for 5 min, as indicated, and lysates (200 μg of protein/sample) were immunoprecipitated with insulin receptor α-subunit antibodies, followed by blotting with phosphotyrosine antibodies (p-Tyr). (C) Alternatively, the cell lysates were immunoprecipitated with IRS-1 or IRS-2 antibodies, followed by blotting with phosphotyrosine, IRS-1, or IRS-2 antibodies, as indicated. The blots were revealed by ECL and autoradiography. The autoradiographs shown are representative of four (A) and three (B and C) independent experiments.
FIG. 2.
Insulin-proliferative and metabolic actions in L6Δ43 cells. (A) L6, L6hIR, and L6Δ43 myoblasts (clones C1, C2, and C8) were stimulated with 100 nM insulin for 10 min as indicated and then solubilized as described in Materials and Methods. Cell lysates (50 μg of protein/sample) were subjected to Western blotting (WB) with phospho-ERK (p-ERK1/2), followed by reblotting with ERK (ERK 1/2) antibodies. Filters were revealed by ECL and autoradiography. The autoradiographs shown are representative of four independent experiments. (B to D) The cells were incubated with 100 nM insulin for 12 h (B) or 10 min (C and D) and assayed for thymidine incorporation into nuclei, 2-deoxy-d-glucose uptake, or glycogen synthase activity as described in Materials and Methods. The bars represent the mean values plus standard deviations of data from three (B), five (C), and four (D) independent experiments, each in triplicate.
Insulin signal transduction in L6Δ43 cells.
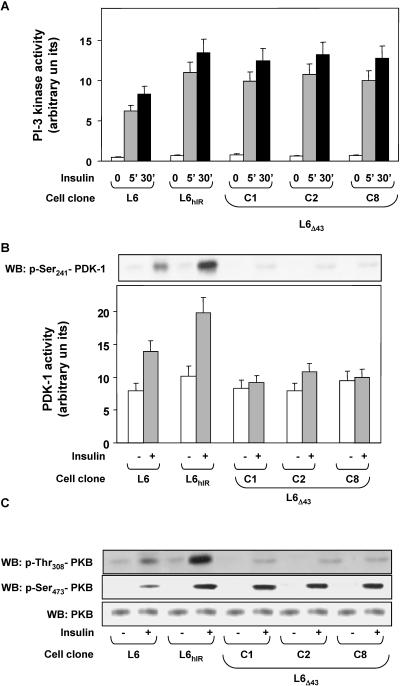
To identify the molecular events involved in the defective activation of glucose utilization in the L6Δ43 cells, we compared PI 3-K activities in L6Δ43 and in L6 and L6hIR cells, as PI 3-K is a major mediator conveying insulin signals to the glucose utilization machinery. Furthermore, there is evidence that PI 3-K may undergo activation upon binding to the insulin receptor C terminus, at least in certain cell types (25, 34, 41). However, we found no significant difference in PI 3-K activity in the L6Δ43 and L6hIR cells, whether in the absence or in the presence of insulin (Fig. 3A).
FIG. 3.
hIRΔ43 signaling in L6 skeletal-muscle cells. (A and B) L6, L6hIR, and L6Δ43 myoblasts (C1, C2, and C8 clones) were stimulated with 100 nM insulin for 10 min (B) or the indicated times (A) and then assayed for PI 3-K or PDK-1 activities as described in Materials and Methods. The bars represent the mean values plus standard deviations of data from three (A) and four (B) independent experiments, in triplicate. (B, top) Alternatively, the cells were lysed and Western blotted (WB) with specific phospho-Ser241 (p-Ser241) antibodies. The blots were revealed by ECL and autoradiography. The blot shown is representative of three independent experiments. (C) The cells were exposed to 100 nM insulin for 10 min and then solubilized as described in Materials and Methods. Cell lysates (50 μg of protein/sample) were blotted with specific phospho-threonine308-PKB antibodies (p-Thr308-PKB) and then reblotted with phospho-Ser473-PKB (p-Ser473-PKB) antibodies. To ensure equal Akt transfer, the filters were further blotted with PKB antibodies (PKB). The filters were revealed by ECL and autoradiography. The autoradiographs shown are representative of four independent experiments.
Basal PDK-1 activity also showed no significant difference in cells expressing the wild-type insulin receptor or the hIRΔ43 (Fig. 3B). Interestingly however, insulin stimulation of PDK-1 was decreased by >80% in L6Δ43 compared to control cells, accompanied by almost complete inhibition of insulin-induced phosphorylation of PDK-1 at Ser241. Western blots with specific phospho-Akt/PKB antibodies revealed that the impaired activation of PDK-1 in the L6Δ43 cells was paralleled by an almost complete lack of insulin phosphorylation of the Thr308 PDK-1 site on Akt/PKB (Fig. 3C). Unlike that of Thr308, insulin-induced phosphorylation of the Ser473 residue of Akt/PKB was unchanged in the L6Δ43 compared to the L6hIR cells. Control blots with Akt/PKB antibodies revealed no difference in the total Akt/PKB levels in these assays.
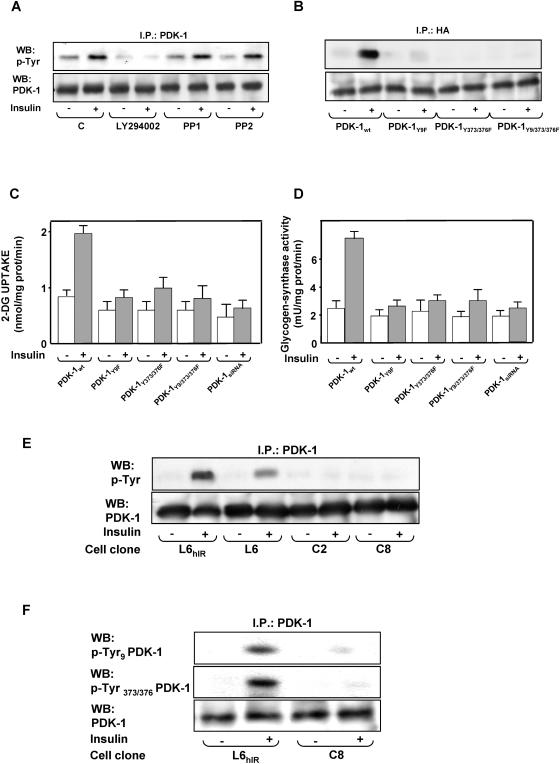
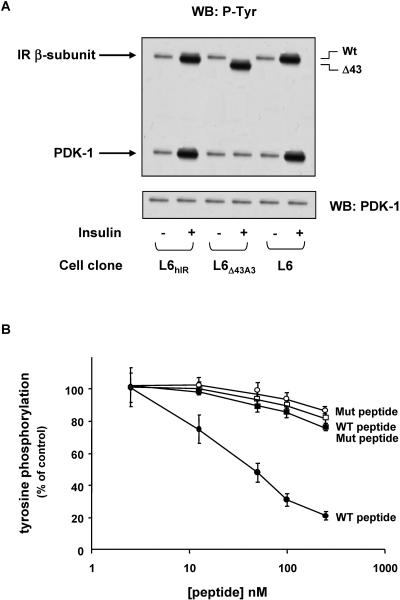
In L6hIR cells, PDK-1 precipitation of the cell lysates, followed by blotting them with phosphotyrosine antibodies, showed a 2.5-fold increase in tyrosine phosphorylation of PDK-1 in response to insulin (Fig. 4A). As in the case of PDK-1 activity (not shown), the effect of insulin on PDK-1 tyrosine phosphorylation was abolished by pretreating the cells with the PI 3-K inhibitor LY294002, though not with the Src kinase inhibitors PP1 and PP2. As previously reported in HEK293 cells (30), in the L6 cells, mutant PDK-1 featuring the substitution of Tyr9, Tyr373, and Tyr376 or the simultaneous replacement of these residues with Phe showed very little phosphorylation in response to insulin (Fig. 4B). These decreased phosphorylations were paralleled by >80%-reduced insulin activation of 2-deoxy-d-glucose uptake and glycogen synthase (Fig. 4C and D). Comparable expression levels for the wild-type and the mutant PDK-1 transfectants were achieved in the L6 cells, implying a major role of PDK-1 tyrosine phosphorylation in the insulin metabolic effect. Treatment of the cells with PDK-1 small interfering RNAs (siRNAs) (43) decreased PDK-1 by 80% (data not shown) and simultaneously impaired insulin action on glucose uptake and glycogen synthase, just as in cells expressing the Tyr→Phe-substituted PDK-1 (Fig. 4C and D). Furthermore, little tyrosine phosphorylation of PDK-1 was detected in L6Δ43 cells, whether in the absence or in the presence of insulin (Fig. 4E). Based on Western blotting with specific phosphotyrosine PDK-1 antibodies, this decreased phosphorylation was accompanied by grossly reduced phosphorylation at Tyr9, Tyr373, and Tyr376 (Fig. 4F). The difference with the tyrosine phosphorylation of PDK-1 occurring in cells expressing the wild-type insulin receptor was accompanied by no change in the expression levels of PDK-1 detected in the two cell types, indicating that the insulin receptor C terminus is involved in insulin activation and tyrosine phosphorylation of PDK-1 through a PI 3-K-dependent, but Src-K-independent, mechanism.
FIG. 4.
Insulin effect on PDK-1 tyrosine phosphorylation. (A and B) L6hIR myoblasts (A) and L6 myoblasts expressing hemagglutinin (HA)-tagged wild-type PDK-1or the indicated PDK-1 mutants (B) were preincubated in the absence or the presence of LY294002 (100 μM for 15 min) or PP1 or PP2 (5 μM for 2 h). Insulin (100 nM) was added to the incubation medium for another 10 min. The cells were then solubilized, and equal amounts of proteins (200 μg) were precipitated (I.P.) with PDK-1 or HA antibodies, as indicated, and Western blotted (WB) with either phosphotyrosine (p-Tyr) or PDK-1 antibodies as described in Materials and Methods. The blots were revealed by ECL and autoradiography. The autoradiographs shown are representative of four (A) and three (B) independent experiments. (C and D) L6 myoblasts expressing either the wild type or the mutant PDK-1 or transfected with a specific PDK-1 siRNA were incubated with 100 nM insulin for 10 min and assayed for 2-deoxy-d-glucose uptake (left) and glycogen synthase activity (right) as described in Materials and Methods. The bars represent the mean values plus standard deviations of data from three independent experiments, each in triplicate. (E and F) L6hIR myoblasts were stimulated with insulin as in panel A and solubilized, and cell proteins (200 μg) were precipitated with PDK-1 antibodies, followed by Western blotting with phosphotyrosine (p-Tyr) or PDK-1 antibodies (E) or with specific pTyr9 or pTyr373/376 antibodies (F), as indicated. The blots were revealed by ECL and autoradiography. The autoradiographs shown are representative of four (E) and three (F) independent experiments.
Cell-surface recruitment of PDK-1 in L6hIRΔ43 cells.
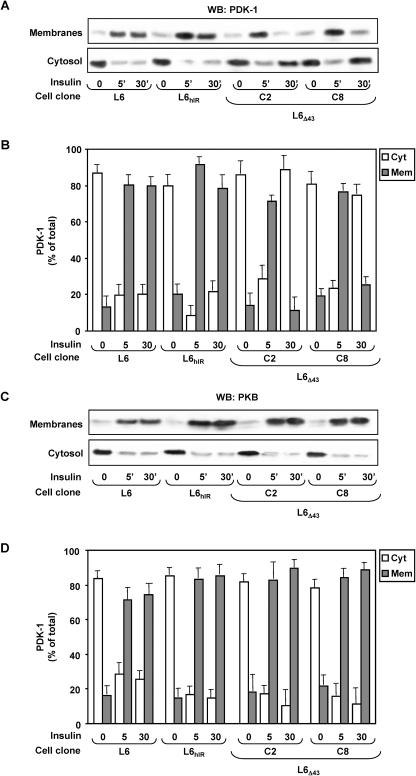
We then aimed at identifying the molecular mechanisms leading to reduced insulin-dependent activation and tyrosine phosphorylation of PDK-1 in the L6Δ43 cells, despite the normal PI 3-K activation. We have therefore hypothesized that the ability of PDK-1 to translocate and/or to localize in the plasma membrane is impaired in the L6Δ43 cells. We stimulated the L6Δ43 and the control cells with insulin for different times and then blotted purified plasma membrane and cytosolic fractions from these cells with PDK-1 antibodies (Fig. 5A and B). In the absence of insulin, PDK-1 was mainly detected in the cytosolic rather than in the plasma membrane fractions of these cells, with no difference in the total levels in each of the two cell types. Upon insulin incubation for 5 min, the plasma membrane content of PDK-1 was significantly reduced in the L6Δ43 compared to both the L6hIR and the L6 cells (P < 0.01). This reduction corresponded to a similarly sized increase in the cytosolic PDK-1 in the L6Δ43 cells. However, upon 30 min of insulin stimulation, almost 80% of the total cellular PDK-1 was localized in the plasma membrane in the control cells, while little PDK-1 was present in plasma membranes from the L6Δ43 cells, as almost all of it was detected in the cytosol. In these same experiments, the different plasma membrane persistence of PDK-1 upon insulin stimulation was not accompanied by changes in the total levels of PDK-1 in the two cell types (data not shown). At variance with PDK-1, the translocation of Akt/PKB to the plasma membrane in response to insulin occurred almost identically in both the control and the L6Δ43 cells (Fig. 5C and D).
FIG. 5.
Insulin effect on PDK-1 and Akt plasma membrane translocation in L6Δ43 cells. L6, L6hIR, and L6Δ43 myoblasts were incubated with 100 nM insulin for the indicated times, followed by cell subfractionation as described in Materials and Methods. Equal amounts of proteins (35 μg) from the purified plasma membrane (Mem) or the cytosolic (Cyt) fractions were then assayed by Western blotting (WB) with PDK-1 (A) or PKB (C) antibodies. The blots were revealed by ECL and autoradiography and subjected to densitometric analysis. The bars represent the mean values plus standard deviations of data from four (B) and three (D) independent experiments. Representative autoradiographs are also shown.
Insulin receptor interaction with PDK-1.
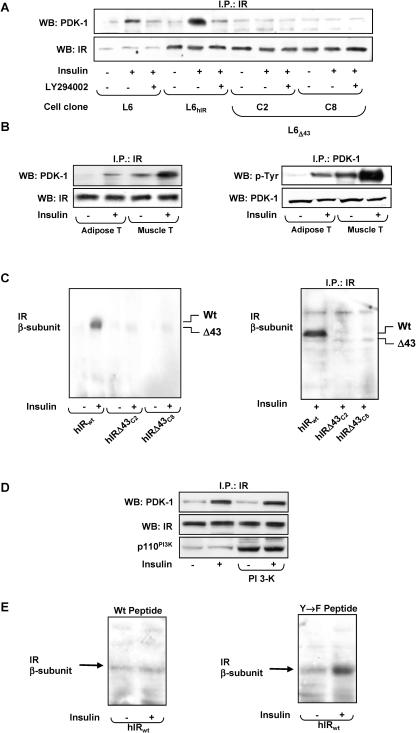
To clarify the mechanism leading to the faster disappearance of PDK-1 from the membrane of insulin-exposed L6Δ43 cells, we compared PDK-1 interaction with the insulin receptor in L6Δ43 and L6hIR cells. To this end, the cells were stimulated with insulin and cell lysates were immunoprecipitated with IR antibodies and blotted with PDK-1 antibodies. In these experiments, PDK-1 coprecipitated with insulin receptors in both L6hIR and L6 cells (Fig. 6A). The coprecipitation was enhanced by insulin. Similar to tyrosine phosphorylation, PDK-1 coprecipitation with IR was almost completely prevented by LY294002 pretreatment of the cells. Interestingly, there was little PDK-1 coprecipitation with hIRΔ43, whether in the absence or in the presence of insulin. These differences in PDK-1 coprecipitation were accompanied by no significant variations in the amounts of insulin receptors precipitated from the L6Δ43 and the control cells. In vivo, the administration of insulin also led to IR-PDK-1 coprecipitation in lysates from mouse skeletal muscle and adipose tissues (Fig. 6B). As in the L6 cells, these coprecipitations were accompanied by insulin-stimulated tyrosine phosphorylation of PDK-1.
FIG. 6.
PDK-1 interaction with the insulin receptor in L6 cells and in vivo. (A) L6, L6hIR, and L6Δ43 myoblasts were preincubated with 100 μM LY294002 for 15 min; 100 nM insulin was added to the incubation medium for an additional 10 min, where indicated. The cells were then solubilized, and equal amounts of proteins (200 μg) were immunoprecipitated (I.P.) with IR α-subunit antibodies, followed by Western blotting (WB) with PDK-1 or IR α-subunit antibodies. (B) Male C57/BL6 mice fasted for 8 h and then were subjected to intraperitoneal fast-acting insulin injection (0.15 IU/g). After 5 min, the animals were sacrificed, followed by collection and processing of gastrocnemius muscles and perigonadal fat pads as described in Materials and Methods. The tissues were lysed, precipitated with insulin receptor antibodies, and blotted with either PDK-1 or phosphotyrosine (p-Tyr) antibodies. For control, aliquots of the lysates were also directly blotted with IR α-subunit or PDK-1 antibodies, as indicated. The blots were revealed by ECL and autoradiography. The autoradiograph shown is representative of four independent experiments. (C) (Left) wheat germ agglutinin-purified insulin receptor preparations (25 μg of proteins) were incubated with 100 nM insulin for 30 min as indicated and then blotted on nitrocellulose filters. Alternatively (right), immunoprecipitated insulin receptor α-subunits from insulin-stimulated L6hIR and L6Δ43 cells were used. Filters were probed with biotinylated recombinant PDK-1 and revealed by autoradiography. The autoradiographs shown are representative of four independent experiments. (D) L6hIR myoblasts were transiently transfected with a cDNA encoding the p110 catalytic subunit of PI 3-K and incubated with 100 nM insulin for 10 min, as indicated. The cells were then solubilized and immunoprecipitated with insulin receptor antibodies, followed by blotting with either PDK-1 or insulin receptor antibodies (top and middle). For control, aliquots of the lysates were directly blotted with PI 3-K antibodies (bottom). The blots were revealed by ECL and autoradiography. The autoradiograph shown is representative of three independent experiments. (E) Immunoprecipitated human insulin receptors were blotted on nitrocellulose filters, and the blots were incubated for another 10 min with either the phosphorylated Δ43 or the Y→F peptide, as indicated. The blots were then probed with biotinylated recombinant PDK-1 and revealed by autoradiography. The autoradiograph shown is representative of three independent experiments.
We also looked for direct PDK-1 interaction with the insulin receptor. WGA-purified wild-type and truncated receptors were incubated with insulin, blotted on nitrocellulose filters, and then incubated with biotinylated recombinant PDK-1. Very little PDK-1 binding to wild-type insulin receptor beta subunits was revealed in the absence of insulin stimulation (Fig. 6C). However, the exposure to insulin induced a >10-fold increase in wild-type insulin receptor-bound PDK-1. The effect of insulin was almost completely absent when the IRΔ43 was probed. Similar results were obtained using immunoprecipitated insulin receptors (Fig. 6C, right).
PI3-K activation by insulin might be sufficient to induce PDK-1 binding to the IR C terminus. Alternatively, phosphorylation of the IR C-terminal tyrosines may also be necessary. To evaluate these possibilities, we first expressed the active PI3-K catalytic subunit in L6hIR cells. Consistent with previous reports (23), the constitutively active PI3-K increased intracellular phosphoinositide levels by >20-fold (data not shown). However, PDK-1 coprecipitation with the IR was unaffected in L6hIR cells expressing the constitutively active PI3-K (Fig. 6D).
We then generated a synthetic peptide corresponding to the IR C-terminal 43 amino acids (amino acids 1340 to 1382; Δ43 peptide) and used Sepharose-bound IR kinase to phosphorylate the peptide. Mass spectrometry analysis revealed that peptide phosphorylation occurred at tyrosines 1354 and 1360 (data not shown). In overlay blot assays, the preincubation of immobilized IR with the phosphorylated peptide inhibited the subsequent binding of PDK-1 (Fig. 6E). At variance, a similar peptide featuring tyrosine 1354 and 1360 replacement with phenylalanine (Y→F peptide) exhibited no such inhibitory effect. It appeared, therefore, that phosphorylation of IR C-terminal tyrosines is necessary to enable PDK-1 binding, while activation of PI3-K is not sufficient. Furthermore, in vitro, insulin-activated IRΔ43 induced no significant tyrosine phosphorylation of L6 cell-immunoprecipitated PDK-1 (Fig. 7A). In this same experiment, insulin incubation of wild-type insulin receptors, from either the L6 or the L6hIR cells, caused a fivefold increase in PDK-1 phosphorylation. The different abilities of IRΔ43 and wild-type IR to phosphorylate PDK-1 was independent of the number of these receptors and of their autophosphorylation levels, as well as of the amount of PDK-1, in the assay. Addition to the phosphorylation reactions of the Δ43 phosphopeptide at increasing concentrations had no effect on IR phosphorylation but caused a dose-dependent reduction of PDK-1 phosphorylation (Fig. 7B). As in the case of PDK-1-IR interaction, the Y→F peptide had no such effect.
FIG. 7.
In vitro phosphorylation of PDK-1 by insulin receptor. (A) L6hIR and L6Δ43 myoblasts were solubilized, and insulin receptors were partially purified by wheat germ agglutinin chromatography. Equal amounts of purified receptors were then stimulated with 100 nM insulin for 10 min, as indicated, and incubated with immunoprecipitated PDK-1 preparations from untransfected L6 myoblasts as described in Materials and Methods. The proteins were blotted with phosphotyrosine antibodies (P-Tyr), and the identities of IR and PDK-1 bands were assessed by Mr and confirmed by reblotting the filters with IR and PDK-1 antibodies, as also shown on the bottom, to ensure equal amounts of PDK-1 in each assay. The blots were revealed by ECL and autoradiography. The autoradiographs shown are representative of four independent experiments. WB, Western blotting; Wt, wild type. (B) WGA-purified hIRs were stimulated with 100 nM insulin and incubated with recombinant PDK-1 in the presence of the indicated concentrations of either the Δ43 or the Y→F peptide, as indicated. The proteins were blotted with phosphotyrosine antibodies and revealed by ECL and autoradiography. Quantitation of the IR beta subunit (open symbols) and PDK-1 bands (filled symbols) was achieved by laser densitometry. The data points are the means ± standard deviations of four independent experiments. Mut, mutant; WT, wild type.
Inhibition of PDK-1 interaction with insulin receptor C terminus.
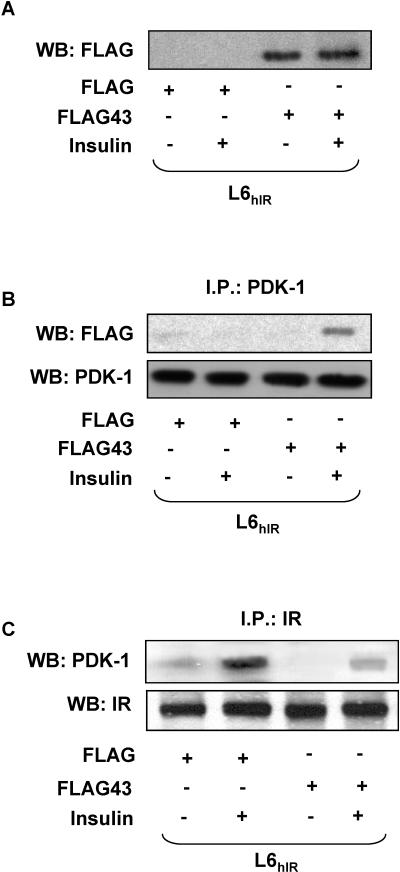
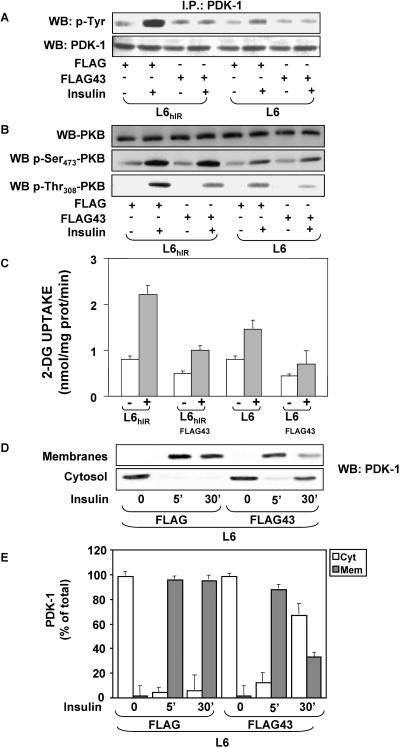
Impaired interaction with IRΔ43 may determine the reduced cell surface accumulation of PDK-1 upon insulin stimulation and its faster cytosolic retrotranslocation. To test this further hypothesis, we generated a FLAG-tagged cDNA encoding amino acids 1340 to 1382 of the insulin receptor COOH terminus. Upon transfection in L6hIR cells, this cDNA expressed a peptide corresponding to the 43-amino-acid C-terminal fragment, which is truncated in IRΔ43 (FLAG43 peptide). By Western blotting with FLAG antibodies, this peptide was detected as a 5-kDa band, while FLAG alone was undetectable (Fig. 8A). Precipitation of insulin-exposed cells expressing the FLAG43 peptide with PDK-1 antibodies, followed by blotting with FLAG antibodies, revealed PDK-1 coprecipitation with the FLAG43 peptide (Fig. 8B). No PDK-1 coprecipitation occurred in lysates from unstimulated cells. No coprecipitation was detected in cells expressing FLAG alone, whether in the absence or the presence of insulin. Importantly, in lysates from cells expressing the FLAG43 peptide, the insulin-induced coprecipitation of PDK-1 with the wild-type insulin receptor was almost threefold reduced compared to cells expressing FLAG alone (Fig. 8C), suggesting that the FLAG43 peptide displaced PDK-1 from the insulin receptor. Similar results were obtained in L6 cells expressing only endogenous insulin receptors (data not shown). Consistently, expression of the FLAG43 peptide reduced insulin-dependent tyrosine phosphorylation of PDK-1 by 10-fold (Fig. 9A). Based on Western blotting with phosphospecific antibodies, cells expressing the FLAG43 peptide also revealed a >2-fold reduction in insulin-stimulated phosphorylation of Akt/PKB at the Thr308, but not the Ser473, site compared to the cells expressing FLAG alone (Fig. 9B). This reduced phosphorylation was independent of the levels of Akt/PKB, indicating that expression of the FLAG peptide may displace PDK-1 from the insulin receptor, impairing PDK-1 phosphorylation and the subsequent phosphorylation of Akt/PKB on Thr308. Consistently, expression of the FLAG43 peptide in L6hIR cells reduced insulin stimulation of 2-deoxy-d-glucose uptake to levels comparable to those measured in the L6Δ43 cells (Fig. 9C).
FIG. 8.
Expression of FLAG43 peptide in L6 cells. (A) L6hIR cells were transiently transfected with 5 μg of the pcDNA3-FLAG expression vector encoding amino acids 1340 to 1382 of the insulin receptor COOH terminus (FLAG43) or with the empty vector (FLAG). The cells were stimulated with 100 nM insulin for 10 min as indicated and then solubilized, and equal amounts of proteins (50 μg) were blotted (WB) with FLAG antibodies. (B) Aliquots of the same lysates (200 μg of proteins) were immunoprecipitated (I.P.) with PDK-1 antibodies and blotted with FLAG antibodies. To ensure equal amounts of PDK-1 in each sample, filters were reblotted with PDK-1 antibodies. (C) Alternatively, the cell lysates were immunoprecipitated with IR α-subunit antibodies, followed by blotting with PDK-1 antibodies. The filters were further blotted with IR α-subunit antibodies to ensure equal amounts of insulin receptor in each sample. The filters were revealed by ECL and autoradiography. The autoradiographs shown are representative of four (A and C) and three (B) independent experiments.
FIG. 9.
Effect of FLAG43 peptide on PDK-1 activity and membrane translocation. (A) L6 and L6hIR cells were transiently transfected with 5 μg of the pcDNA3-FLAG expression vector encoding amino acids 1340 to 1382 of the insulin receptor COOH terminus (FLAG43) or with the empty vector (FLAG). The cells were stimulated with 100 nM insulin for 10 min as indicated and then solubilized, and equal amounts of proteins (200 μg) were immunoprecipitated (I.P.) with PDK-1 antibodies and blotted (WB) with phosphotyrosine antibodies (p-Tyr). To ensure equal amounts of PDK-1 in each sample, filters were reblotted with PDK-1 antibodies. (B) Alternatively, aliquots of the cell lysates (50 μg of proteins) were directly blotted with specific antibodies to either the Ser437 or the Thr308 residue of PKB (p-Ser437- and p-Thr308-PKB). For controls, the lysates were also reblotted with PKB antibodies. Filters were revealed by ECL and autoradiography. The autoradiographs shown are representative of three (A) and four (B) independent experiments. (C) FLAG43- and FLAG-transfected cells were incubated with 100 nM insulin for 10 min and assayed for 2-deoxy-d-glucose uptake as described in Materials and Methods. The bars represent the mean values plus standard deviations (SD) of data from three independent experiments, each in triplicate. (D and E) Cells transfected with FLAG43 or the empty vector were stimulated with 100 nM insulin for the indicated times, followed by cell subfractionation as described in Materials and Methods. Equal amounts of protein from the membrane (35 μg) or the cytosolic (35 μg) fraction were then assayed by Western blotting with PDK-1 antibodies. The blots were revealed by ECL and autoradiography and quantitated by densitometric analysis. The autoradiographs shown in panel D are from a representative experiment. The bars (E) represent the means ± SD of four independent experiments.
As in L6Δ43 cells, 5-min stimulation with insulin induced a 25%-reduced plasma membrane translocation of PDK-1 in L6 cells expressing the FLAG43 peptide compared to cells expressing FLAG alone (Fig. 9D and E). Furthermore, PDK-1 was no longer detected in the plasma membranes of cells expressing the FLAG43 peptide after 30 min of insulin stimulation. PDK-1 remained easily detectable in the control cells, however. Similar results were obtained by comparing cells expressing the FLAG43 peptide and the L6hIR cells (data not shown), indicating that binding to the insulin receptor C terminus is necessary to enable normal PDK-1 membrane localization and activation in response to insulin.
DISCUSSION
PDK-1 activation is a key event in the intracellular signaling triggered by a number of hormones, including insulin (3). There is evidence that insulin induces tyrosine phosphorylation of PDK-1 (30), which may contribute to its subcellular redistribution and activation. However, the molecular mechanism through which insulin triggers PDK-1 activation, as well as the kinase responsible for PDK-1 phosphorylation in the insulin-stimulated cells, remains elusive. In the present work, we report that insulin induces phosphorylation of PDK-1 on tyrosines 9 and 373/376 and activation of PDK-1 in cultured L6 skeletal-muscle cells, as well as in mouse skeletal muscle and adipose tissue. Consistent with previous studies by Park et al. (30), these tyrosine phosphorylation events are necessary for insulin metabolic action, as expression of Tyr→Phe-substituted PDK-1 blocks insulin stimulation of both glucose uptake and glycogen synthase in L6 cells, like treatment with a specific PDK-1 siRNA. In the cell extracts from these cells, insulin also induces the coprecipitation of insulin receptors with PDK-1. The coprecipitation did not occur in cell extracts expressing the hIRΔ43 C-terminally truncated receptor, indicating that PDK-1 binds to the active insulin receptor kinase and that the 43 C-terminal amino acids of the insulin receptor are necessary for the binding to occur.
Consistent with earlier findings in different (26), though not all, cell types (29), we have also shown that the hIRΔ43 receptor is unable to transduce the insulin stimulatory effect on glucose transport and glycogen synthase in L6 cells while conveying proliferative stimuli. In parallel, there was little insulin-dependent tyrosine phosphorylation and activation of PDK-1 in L6 cells expressing hIRΔ43 receptors. Thus, binding to the active insulin receptor kinase is accompanied by PDK-1 tyrosine phosphorylation and activation.
Src family tyrosine kinases have been implicated in insulin signaling and shown to phosphorylate PDK-1 on tyrosine in certain cell types (20, 30). However, Src kinases do not mediate insulin action on PDK-1 tyrosine phosphorylation in L6 cells, as Src kinase inhibitors have no effect on PDK-1 phosphorylation by insulin. The insulin receptor kinase itself appears to phosphorylate on tyrosine and to activate PDK-1 in skeletal-muscle cells. In fact, (i) as in the intact cells, the wild-type but not the hIRΔ43 receptors bind and phosphorylate recombinant PDK-1 in vitro, and (ii) a peptide corresponding to the 43-amino-acid sequence of the insulin receptor C terminus (Δ43 peptide) simultaneously prevents insulin receptor interaction and phosphorylation of PDK-1 and PDK-1 activation, both in vitro and in intact cells. The additional finding that the Δ43 peptide coprecipitates with PDK-1 only in extracts from insulin-stimulated cells indicates that PDK-1 interaction requires phosphorylation of the insulin receptor C-terminal tyrosines. Indeed, we showed that (i) Tyr→Phe substitution in the Δ43 peptide abolished its ability to inhibit both the insulin-induced IR-PDK-1 interaction and PDK-1 tyrosine phosphorylation and (ii) the Δ43 peptide undergoes tyrosine phosphorylation by insulin (data not shown).
Previous reports demonstrated that the binding of PI 3-K lipids to the PH domain of PDK-1 targets PDK-1 to the plasma membrane, enabling PDK-1 activation and glucose uptake (2, 35, 39). How PDK-1 membrane interaction affects kinase activity is poorly defined, however. As previously demonstrated in other cells, we reported that blocking of PI 3-K in insulin-exposed L6 cells prevents PDK-1 membrane translocation and activation. In addition to inducing PDK-1 activity, however, we further showed that activated insulin receptors anchor the membrane-targeted PDK-1 at the cell surface. Indeed, (i) membrane-targeted PDK-1 in L6 cells expressing hIRΔ43 receptors retrotranslocate to the cytosol significantly faster than in cells expressing wild-type receptors, and (ii) blocking of wild-type insulin receptor binding with the Δ43 peptide induces fast cytosolic translocation of PDK-1 in insulin-stimulated L6 cells, accompanied by reduced insulin-stimulated metabolic effects. When cells are exposed to multiple hormones in the extracellular fluid, the persistence of PDK-1 in the membrane due to activated insulin receptors may prolong PDK-1 activity. Indeed, PDK-1 activation in response to platelet-derived growth factor shows a shorter time course in L6 cells expressing hIRΔ43 than in cells expressing wild-type receptors (data not shown). Thus, PDK-1 membrane anchoring by the insulin receptor may also affect response to hormones other than insulin.
Mutants of PDK-1 unable to translocate to the plasma membrane prevent membrane recruitment and subsequent activation of Akt/PKB in insulin-stimulated cells (2). Based on this finding, it has been proposed that PDK-1 recruits Akt/PKB to the plasma membrane, where the activation of Akt/PKB occurs (14). However, in L6 cells expressing hIRΔ43 receptors, Akt/PKB normally translocates following insulin exposure, despite the lack of PDK-1 activation and the reduced presence of PDK-1 in the membrane. It is possible that insulin activation of the endogenous receptors in the hIRΔ43 transfectants is sufficient to allow maximal translocation of Akt/PKB. Alternatively, PI 3-K and/or PDK-2 activation may be sufficient for Akt/PKB recruitment in the hIRΔ43-transfected cells. Indeed, previous studies by Williams et al. (45) demonstrated that, in cells lacking PDK-1, growth factor-stimulated phosphorylation of Akt/PKB on Thr308 did not occur, but phosphorylation of Ser473 still remained intact. Consistently, insulin-dependent phosphorylation of Ser473 was unaffected in the hIRΔ43-transfected cells.
In conclusion, we report, for the first time that the insulin receptor kinase binds to and tyrosine phosphorylates PDK-1 in response to insulin (thereby activating PDK-1). Membrane anchoring of PDK-1 through the C-terminal 1340-to-1382 region of the insulin receptor is a crucial step in insulin metabolic action in skeletal-muscle cells and may affect PDK-1 signaling in response to other hormones as well.
Acknowledgments
This work was supported by the European Community's FP6 EUGENE2 (LSHM-CT-2004-512013), grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC) to F.B. and P.F., and the Ministero dell'Università e della Ricerca Scientifica (PRIN) to F.B. and P.F and FIRB RBNE0155LB to F.B. The financial support of Telethon-Italy is gratefully acknowledged.
We also thank D. Liguoro (IEOS, CNR) for technical help.
REFERENCES
- 1.Alessi, D. R., M. T. Kozlowski, Q. P. Weng, N. Morrice, and J. Avruch. 1998. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr. Biol. 8:69-81. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, K. E., J. Coadwell, L. R. Stephens, and P. T. Hawkins. 1998. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr. Biol. 8:684-691. [DOI] [PubMed] [Google Scholar]
- 3.Belham, C., S. Wu, and J. Avruch. 1999. Intracellular signalling: PDK-1—a kinase at the hub of things. Curr. Biol. 9:R93-R96. [DOI] [PubMed] [Google Scholar]
- 4.Cantrell, D. A. 2001. Phosphoinositide 3-kinase signalling pathways. J. Cell Sci. 114:1439-1445. [DOI] [PubMed] [Google Scholar]
- 5.Caruso, M., C. Miele, F. Oriente, M. A. Maitan, G. Bifulco, F. Andreozzi, G. Condorelli, P. Formisano, and F. Beguinot. 1999. In L6 skeletal muscle cells, glucose induces cytosolic translocation of protein kinase C-alpha and trans-activates the insulin receptor kinase. J. Biol. Chem. 274:28637-28644. [DOI] [PubMed] [Google Scholar]
- 6.Caruso, M., C. Miele, P. Formisano, G. Condorelli, G. Bifulco, A. Oliva, R. Auricchio, G. Riccardi, B. Capaldo, and F. Beguinot. 1997. In skeletal muscle, glucose storage and oxidation are differentially impaired by the IR1152 mutant receptor. J. Biol. Chem. 272:7290-7297. [DOI] [PubMed] [Google Scholar]
- 7.Caruso, M., M. A. Maitan, G. Bifulco, C. Miele, G. Vigliotta, F. Oriente, P. Formisano, and F. Beguinot. 2001. Activation and mitochondrial translocation of protein kinase Cδ are necessary for insulin stimulation of pyruvate dehydrogenase complex activity in muscle and liver cells. J. Biol. Chem. 276:45088-45097. [DOI] [PubMed] [Google Scholar]
- 8.Casamayor, A., N. A. Morrice, and D. R. Alessi. 1999. Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem. J. 342:287-292. [PMC free article] [PubMed] [Google Scholar]
- 9.Chou, M. M., W. Hou, J. Johnson, L. K. Graham, M. H. Lee, C. S. Chen, A. C. Newton, B. S. Schaffhausen, and A. Toker. 1998. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr. Biol. 8:1069-1077. [DOI] [PubMed] [Google Scholar]
- 10.Dong, L. Q., and F. Liu. 2005. PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am. J. Physiol. Endocrinol. Metab. 289:E187-E196. [DOI] [PubMed] [Google Scholar]
- 11.Dong, L. Q., R. Zhang, P. Langlais, H. He, M. Clark, L. Zhu, and F. Liu. 1999. Primary structure, tissue distribution, and expression of mouse phosphoinositide-dependent protein kinase-1, a protein kinase that phosphorylates and activates protein kinase C. J. Biol. Chem. 274:8117-8122. [DOI] [PubMed] [Google Scholar]
- 12.Dutil, E. M., A. Toker, and A. C. Newton. 1998. Regulation of conventional protein kinase C isozymes by phosphoinositide-dependent kinase 1 (PDK-1). Curr. Biol. 8:1366-1375. [DOI] [PubMed] [Google Scholar]
- 13.Fields, G. B., and R. L. Noble. 1990. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 35:161-214. [DOI] [PubMed] [Google Scholar]
- 14.Filippa, N., C. L. Sable, B. A. Hemmings, and E. Van Obberghen. 2000. Effect of phosphoinositide-dependent kinase 1 on protein kinase B translocation and its subsequent activation. Mol. Cell. Biol. 20:5712-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filippa, N., C. L. Sable, C. Filloux, B. Hemmings, and E. Van Obberghen. 1999. Mechanism of protein kinase B activation by cyclic AMP-dependent protein kinase. Mol. Cell. Biol. 19:4989-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiory, F., F. Oriente, C. Miele, C. Romano, A. Trencia, A. T. Alberobello, I. Esposito, R. Valentino, F. Beguinot, and P. Formisano. 2004. Protein kinase C-zeta and protein kinase B regulate distinct steps of insulin endocytosis and intracellular sorting. J. Biol. Chem. 279:11137-11145. [DOI] [PubMed] [Google Scholar]
- 17.Formisano, P., F. Oriente, C. Miele, M. Caruso, R. Auricchio, G. Vigliotta, G. Condorelli, and F. Beguinot. 1998. In NIH-3T3 fibroblasts, insulin receptor interaction with specific protein kinase C isoforms controls receptor intracellular routing. J. Biol. Chem. 273:13197-13202. [DOI] [PubMed] [Google Scholar]
- 18.Formisano, P., K. J. Sohn, C. Miele, B. Di Finizio, A. Petruzziello, G. Riccardi, L. Beguinot, and F. Beguinot. 1993. Mutation in a conserved motif next to the insulin receptor key autophosphorylation sites de-regulates kinase activity and impairs insulin action. J. Biol. Chem. 268:5241-5248. [PubMed] [Google Scholar]
- 19.Foukas, L. C., and P. R. Shepherd. 2004. Phosphoinositide 3-kinase: the protein kinase that time forgot. Biochem. Soc. Trans. 32:330-331. [DOI] [PubMed] [Google Scholar]
- 20.Grillo, S., T. Gremeaux, A. Casamayor, D. R. Alessi, Y. Le Marchand-Brustel, and J. F. Tanti. 2000. Peroxovanadate induces tyrosine phosphorylation of phosphoinositide-dependent protein kinase-1 potential involvement of src kinase. Eur. J. Biochem. 267:6642-6649. [DOI] [PubMed] [Google Scholar]
- 21.Hresko, R. C., H. Murata, and M. Mueckler. 2003. Phosphoinositide-dependent kinase-2 is a distinct protein kinase enriched in a novel cytoskeletal fraction associated with adipocyte plasma membranes. J. Biol. Chem. 278:21615-21622. [DOI] [PubMed] [Google Scholar]
- 22.Kandel, E. S., and N. Hay. 1999. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp. Cell Res. 253:210-229. [DOI] [PubMed] [Google Scholar]
- 23.Klippel, A., C. Reinhard, W. M. Kavanaugh, G. Apell, M. A. Escobedo, and L. T. Williams. 1996. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol. Cell. Biol. 16:4117-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi, T., and P. Cohen. 1999. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem. J. 339:319-328. [PMC free article] [PubMed] [Google Scholar]
- 25.Levy-Toledano, R., M. Taouis, D. H. Blaettler, P. Gorden, and S. I. Taylor. 1994. Insulin-induced activation of phosphatidyl inositol 3-kinase. Demonstration that the p85 subunit binds directly to the COOH terminus of the insulin receptor in intact cells. J. Biol. Chem. 269:31178-31182. [PubMed] [Google Scholar]
- 26.Maegawa, H., D. A. McClain, G. Freidenberg, J. M. Olefsky, M. Napier, T. Lipari, T. J. Dull, J. Lee, and A. Ullrich. 1988. Properties of a human insulin receptor with a COOH-terminal truncation. II. Truncated receptors have normal kinase activity but are defective in signaling metabolic effects. J. Biol. Chem. 263:8912-8917. [PubMed] [Google Scholar]
- 27.McManus, E. J., B. J. Collins, P. R. Ashby, A. R. Prescott, V. Murray-Tait, L. J. Armit, J. S. Arthur, and D. R. Alessi. 2004. The in vivo role of PtdIns(3,4,5)P(3) binding to PDK1 PH domain defined by knockin mutation. EMBO J. 23:2071-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mora, A., D. Komander, D. M. van Aalten, and D. R. Alessi. 2004. PDK1, the master regulator of AGC kinase signal transduction. Semin. Cell Dev. Biol. 15:161-170. [DOI] [PubMed] [Google Scholar]
- 29.Myers, M. G., J. M. Backer, K. Siddle, and M. F. White. 1991. The insulin receptor functions normally in Chinese hamster ovary cells after truncation of the C terminus. J. Biol. Chem. 266:10616-10623. [PubMed] [Google Scholar]
- 30.Park, J., M. M. Hill, D. Hess, D. P. Brazil, J. Hofsteenge, and B. A. Hemmings. 2001. Identification of tyrosine phosphorylation sites on 3-phosphoinositide-dependent protein kinase-1 and their role in regulating kinase activity. J. Biol. Chem. 276:37459-37471. [DOI] [PubMed] [Google Scholar]
- 31.Prasad, N., R. S. Topping, D. Zhou, and S. J. Decker. 2000. Oxidative stress and vanadate induce tyrosine phosphorylation of phosphoinositide-dependent kinase 1 (PDK1). Biochemistry 39:6929-6935. [DOI] [PubMed] [Google Scholar]
- 32.Piper, R. C., L. J. Hess, and D. E. James. 1991. Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am. J. Physiol. 260:C570-C580. [DOI] [PubMed] [Google Scholar]
- 33.Romanelli, A., V. C. Dreisbach, and J. Blenis. 2002. Characterization of phosphatidylinositol 3-kinase-dependent phosphorylation of the hydrophobic motif site Thr(389) in p70 S6 kinase 1. J. Biol. Chem. 277:40281-40289. [DOI] [PubMed] [Google Scholar]
- 34.Ruderman, N. B., R. Kapeller, M. F. White, and L. C. Cantley. 1990. Activation of phosphatidylinositol 3-kinase by insulin. Proc. Natl. Acad. Sci. USA 87:1411-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakaue, H., A. Nishizawa, W. Ogawa, K. Teshigawara, T. Mori, Y. Takashima, T. Noda, and M. Kasuga. 2003. Requirement for 3-phosphoinositide-kependent kinase-1 (PDK-1) in insulin-induced glucose uptake in immortalized brown adipocytes. J. Biol. Chem. 278:38870-38874. [DOI] [PubMed] [Google Scholar]
- 36.Sarbassov, D. D., D. A. Guertin, S. M. Ali, and D. M. Sabatini. 2005. Phosphorylation and regulation of Akt/PKB by the Rictor-mTOR complex. Science 307:1098-1101. [DOI] [PubMed] [Google Scholar]
- 37.Simpson, I. A., D. R. Yver, P. J. Hissin, L. J. Wardzala, E. Karnieli, L. B. Salans, and S. W. Cushman. 1983. Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim. Biophys. Acta 763:393-407. [DOI] [PubMed] [Google Scholar]
- 38.Storz, P., and A. Toker. 2002. 3′-phosphoinositide-dependent kinase-1 (PDK-1) in PI 3-kinase signaling. Front Biosci. 7:886-902. [DOI] [PubMed] [Google Scholar]
- 39.Toker, A., and A. C. Newton. 2000. Cellular signaling: pivoting around PDK-1. Cell 103:185-188. [DOI] [PubMed] [Google Scholar]
- 40.Vanhaesebroeck, B., and D. R. Alessi. 2000. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346:561-576. [PMC free article] [PubMed] [Google Scholar]
- 41.Van Horn, D. J., M. G. Myers, Jr., and J. M. Backer. 1994. Direct activation of the phosphatidylinositol 3′-kinase by the insulin receptor. J. Biol. Chem. 269:29-32. [PubMed] [Google Scholar]
- 42.Vigliotta, G., C. Miele, S. Santopietro, G. Portella, A. Perfetti, M. A. Maitan, A. Cassese, F. Oriente, A. Trencia, F. Fiory, C. Romano, C. Tiveron, L. Tatangelo, G. Troncone, P. Formisano, and F. Beguinot. 2004. Overexpression of the ped/pea-15 gene causes diabetes by impairing glucose-stimulated insulin secretion in addition to insulin action. Mol. Cell. Biol. 24:5005-5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei, Q., and Y. Xia. 2005. Roles of 3-phosphoinositide-dependent kinase 1 in the regulation of endothelial nitric-oxide synthase phosphorylation and function by heat shock protein 90. J. Biol. Chem. 280:18081-18086. [DOI] [PubMed] [Google Scholar]
- 44.Wick, K. L., and F. Liu. 2001. A new molecular target of insulin action: regulating the pivotal PDK1. Curr. Drug Targets Immune Endocr. Metabol. Disord. 1:209-221. [DOI] [PubMed] [Google Scholar]
- 45.Williams, M. R., J. S. Arthur, A. Balendran, J. van der Kaay, V. Poli, P. Cohen, and D. R. Alessi. 2000. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr. Biol. 10:439-448. [DOI] [PubMed] [Google Scholar]