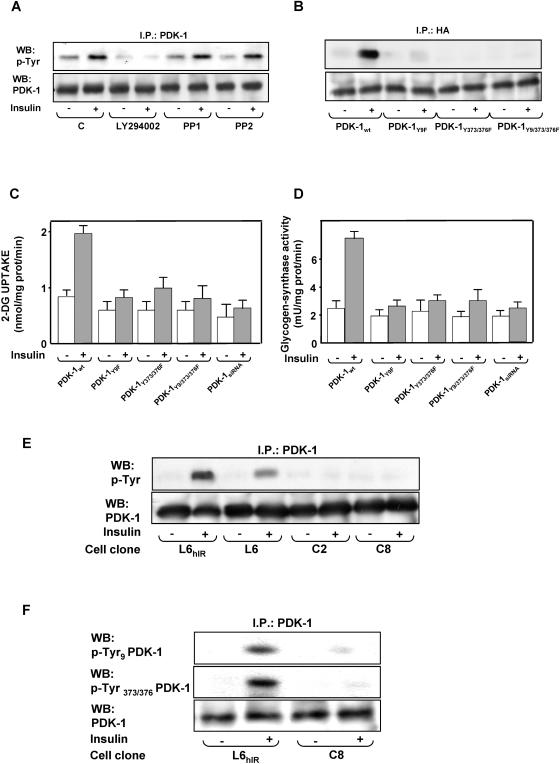
FIG. 4.
Insulin effect on PDK-1 tyrosine phosphorylation. (A and B) L6hIR myoblasts (A) and L6 myoblasts expressing hemagglutinin (HA)-tagged wild-type PDK-1or the indicated PDK-1 mutants (B) were preincubated in the absence or the presence of LY294002 (100 μM for 15 min) or PP1 or PP2 (5 μM for 2 h). Insulin (100 nM) was added to the incubation medium for another 10 min. The cells were then solubilized, and equal amounts of proteins (200 μg) were precipitated (I.P.) with PDK-1 or HA antibodies, as indicated, and Western blotted (WB) with either phosphotyrosine (p-Tyr) or PDK-1 antibodies as described in Materials and Methods. The blots were revealed by ECL and autoradiography. The autoradiographs shown are representative of four (A) and three (B) independent experiments. (C and D) L6 myoblasts expressing either the wild type or the mutant PDK-1 or transfected with a specific PDK-1 siRNA were incubated with 100 nM insulin for 10 min and assayed for 2-deoxy-d-glucose uptake (left) and glycogen synthase activity (right) as described in Materials and Methods. The bars represent the mean values plus standard deviations of data from three independent experiments, each in triplicate. (E and F) L6hIR myoblasts were stimulated with insulin as in panel A and solubilized, and cell proteins (200 μg) were precipitated with PDK-1 antibodies, followed by Western blotting with phosphotyrosine (p-Tyr) or PDK-1 antibodies (E) or with specific pTyr9 or pTyr373/376 antibodies (F), as indicated. The blots were revealed by ECL and autoradiography. The autoradiographs shown are representative of four (E) and three (F) independent experiments.