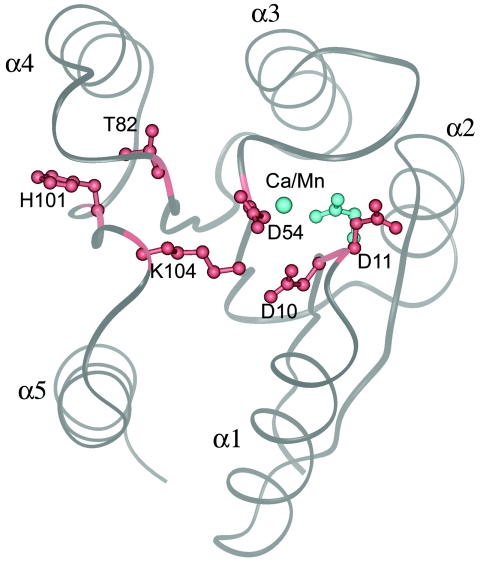
FIG. 2.
A view of Spo0F from the top of the β-sheet, showing the overall fold. The five helices are marked α1 to α5. There are five β-strands (β1 to β5), and each one precedes the corresponding helix; that is, strand β1 is on the N-terminal side of helix α1. Shown in gray is a ribbon representation of the metal-free form of Spo0F depicting the location of six functionally critical residues. The conserved aspartates Asp 10, Asp 11, and Asp 54 form the acidic portion of the active site. A conserved lysine residue, Lys104, points towards the aspartates. Positions 82 and 101 are also normally conserved with a hydroxyl-containing Ser/Thr and an aromatic residue, respectively. In Spo0F, position 82 is a threonine, but 101 is a histidine. These two residues point away from the active site. The orientations of Asp 10, Asp 11, Asp 54, Thr82, His101, and Lys104 are shown in red for the metal-free structure. Upon metal coordination, Asp11 reorients to form the metal coordination site. The location of the metal ion and the new orientation of Asp11 in the metal-bound structures are shown in cyan.