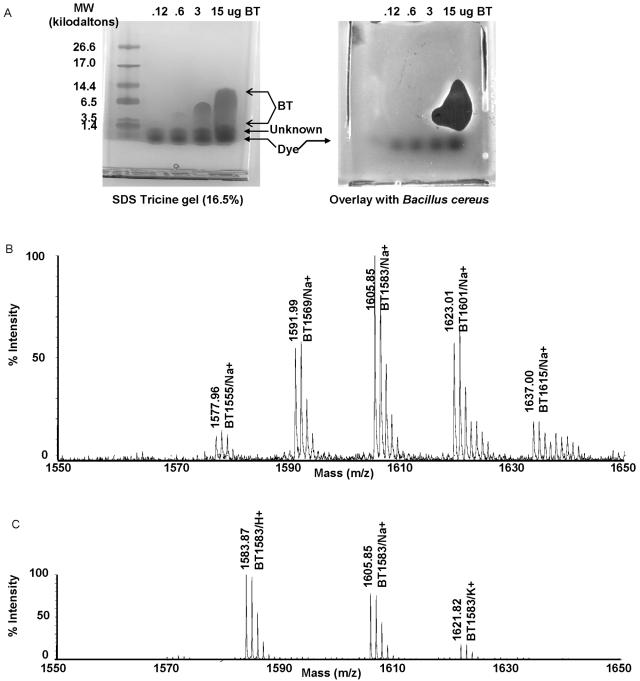
FIG. 1.
Purification of peptide BT. (A) Tricine gel separation of BT and the associated antibiotic activity. Chloroform-treated extracted peptides were separated on a precast 16.5% Tricine gel (purchase from Bio-Rad). One gel was stained with Coomassie blue to show peptide bands. Another gel was overlaid with agar containing B. cereus. Clear zones in the bacterial lawn correspond to the species that collapses into a single band at a molecular weight of ∼1,500. The following molecular mass markers were used: triose phosphate isomerase (26.6 kDa), myoglobin (17.0 kDa), alpha-lactalbumin (14.4 kDa), aprotinin (6.5 kDa), insulin b chain (oxidized) (3.5 kDa), and bacitracin (1.4 kDa). SDS, sodium dodecyl sulfate. (B) Mass spectrometry of chloroform-extracted BT. Chloroform-extracted BT was treated with sodium by addition of sodium chloride and then subjected to mass spectrometry analysis. Five sodium-containing BT isomers (BT1555, BT1571, BT1583, BT1599, and BT1613) were detected and labeled. (C) Mass spectrometry of purified BT1583. Fraction 33 from C18 reverse-phase HPLC was subjected to mass spectrometry analysis. Only protonated, sodium-containing, and potassium-containing BT1583 were detected.