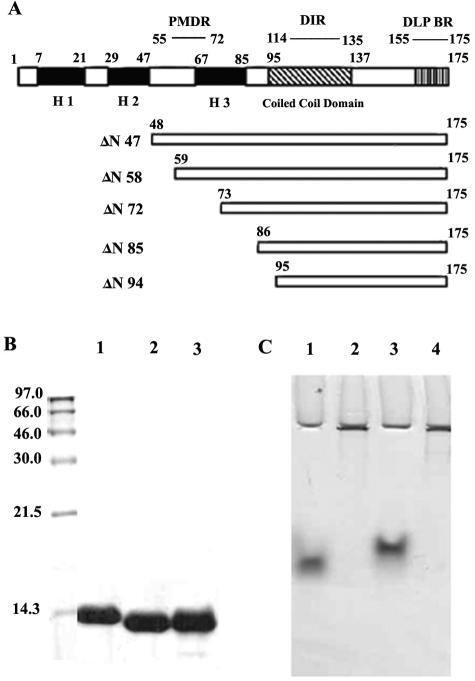
FIG. 1.
(A) Schematic representation of deletion mutants ΔN47, ΔN57, ΔN72, ΔN85, and ΔN94 of NSP4 from rotavirus strain Hg18. PMDR, proximal membrane-destabilizing region; DLP-BR, double-layered particle-binding region. Darkly shaded boxes represent the three N-terminal hydrophobic domains H1, H2, and H3. (B) SDS-PAGE in 16% gel of the purified NSP4ΔN72, NSP4ΔN85, and NSP4ΔN94 mutant proteins. Molecular weights of the markers are indicated to the left of the gel. (C) Native PAGE of NSP4ΔN72, NSP4ΔN85, and NSP4ΔN94 from strains Hg18 and SA11 in an 8% gel. Lane 1, Hg18ΔN94; lane 2, Hg18ΔN72; lane 3, Hg18ΔN85; lane 4, SA11ΔN72. Note that ΔN72 from both the strains remained near the wells.