FIG. 4.
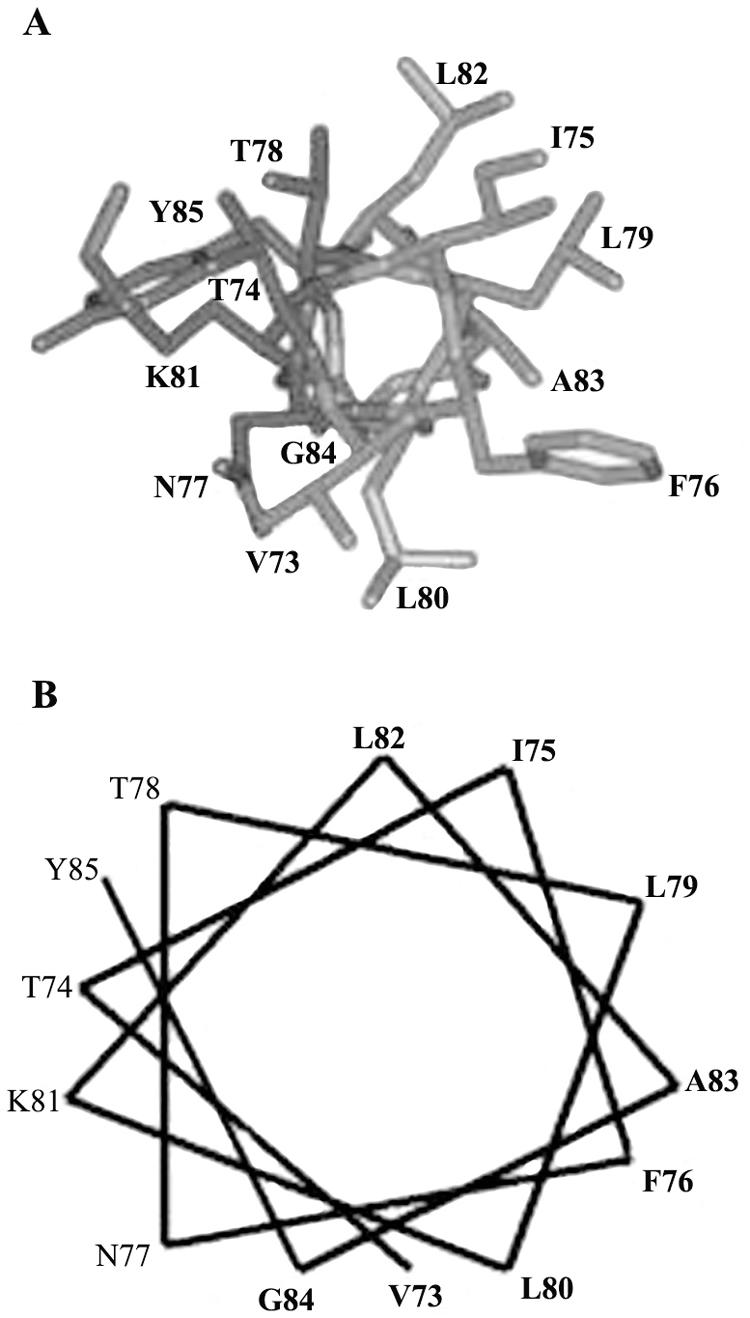
NSP4 aa 73 to 85 are predicted to assume amphipathic α-helical conformation. (A) α-Helical model building of the Hg18 NSP4 peptide sequence (VTIFNTLLKLAGY) from residues 73 to 85 based on the α-helical structures of the aligned peptide segments from human immunodeficiency virus integrase, cytochrome c oxidase, FtsA, and annexin I. (B) Helical wheel representation of amino acid residues 73 to 85 from Hg18 NSP4 as an amphipathic helix. Note the clustering polar amino acids and a single lysine on one face and nonpolar amino acids on the other side of the putative helix.
