FIG. 6.
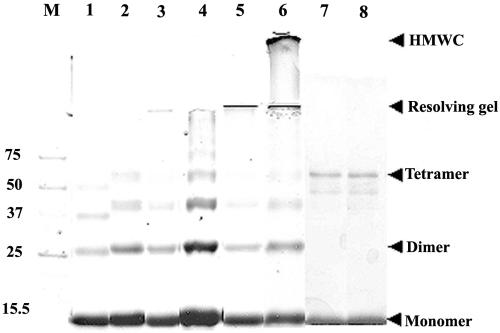
Majority of the HMWC of Hg18ΔN72 or SA11ΔN72 proceed through ordered multimerization of tetramers as demonstrated by glutaraldehyde cross-linking. ΔN85 (lane 1), Hgm1 (lane 2), Hgm15 (lane 3), Hg18ΔN72 (lane 4, 1 h; lane 5, 4 h; lane 6, 12 h) at 5 nmol (at an approximately 600-μg/ml concentration); the oligomeric form of SA11dirm2 (lane 7) at 2 nmol (3 μg/ml in 8 ml for 12 h); and the protein eluting at an apparent molecular weight of 36 to 39 of SA11dirm4 (lane 8) were cross-linked using an equimolar ratio (1:1) of cross-linker to protein for indicated time periods. Note tetramers of all the mutants and a range of multimers proceeding through tetramers of ΔN72 at 1 h of cross-linking (lane 4), HMWC of ΔN72 that barely migrated into the resolving gel (lane 5, 4 h), and HMWC that remained just below the well in the stacking gel (lane 6, 12 h). The start of resolving and stacking gels and the positions of monomeric, dimeric, and tetrameric forms are indicated by arrows. At a high ratio of cross-linker to protein (100:1 or 200:1), the majority of ΔN72 goes into HMWC within 5 min of cross-linking (data not shown). At a 3.0-μg/ml concentration, only tetramers of dirm2 and dirm4 are seen (lanes 7 and 8), but at 10 μg/ml, multimers are also observed (data not shown). M, protein molecular weight markers.