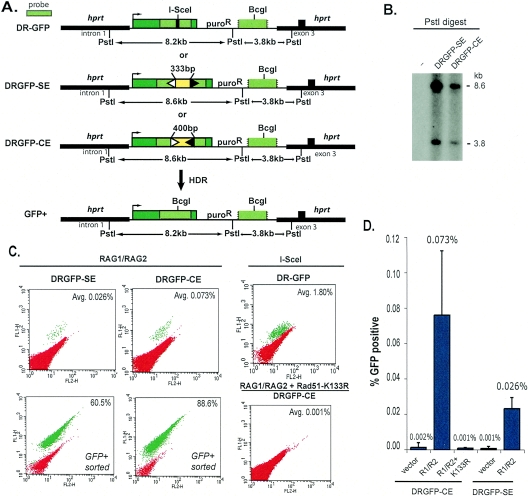
FIG. 1.
Homology-directed repair at RAG-generated breaks. A. HDR reporters for RAG-generated DSBs were designed based on the previously published DR-GFP reporter (37) for the repair of I-SceI endonuclease-generated DSBs. The DRGFP-SE (signal end) and DRGFP-CE (coding end) reporters have two RSS elements, one having a 12-bp spacer (white triangle) and the other a 23-bp spacer (black triangle), which interrupt a GFP gene (green box). The RSS elements are separated by 333 bp (yellow box) and, when cleaved by the RAG proteins, generate a GFP gene with either signal ends (DRGFP-SE) or coding ends (DRGFP-CE). DSB repair by a noncrossover gene conversion with the downstream 5′- and 3′-truncated GFP gene results in a functional GFP gene. Unlike DRGFP-CE, gene conversion at DRGFP-SE requires end processing to remove the RSS elements (see also Fig. 2A). B. Southern analysis demonstrating targeting of the DRGFP-SE and DRGFP-CE reporters to the hprt locus in mouse ES cells. Genomic DNA was digested with PstI, which cleaves once in the reporter targeting fragment. Fragments of 8.6 and 3.8 kb are obtained from hprt−/puroR cells that are appropriately targeted. Lane −, negative control. C. Flow-cytometric analysis of ES cells containing the HDR reporters at the hprt locus after expression of full-length RAG1 and RAG2 in cells containing DRGFP-SE or DRGFP-CE (in the latter case with Rad51-K133R where indicated) or after I-SceI expression in cells containing DR-GFP. The percentage of GFP+ cells obtained from >50,000 analyzed cells is indicated (average from three or more experiments). GFP+ cells arising from RAG1/RAG2 expression were also enriched by flow sorting. D. Frequency of GFP+ cells by flow cytometry in ES cells containing the HDR reporters after transient transfection of either empty vector, full-length RAG1 and RAG2 (R1/R2), or full-length RAG1 and RAG2 and Rad51-K133R (K133R). Error bars indicate 1 standard deviation from the mean.