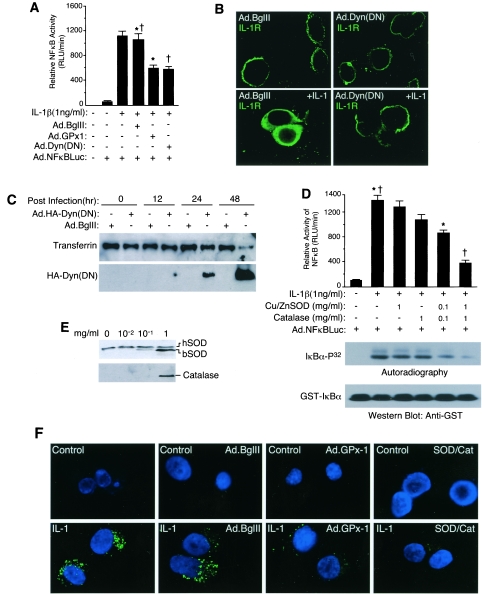
FIG. 1.
Redox regulation of NF-κB activation is dependent on endocytosis. (A and D) MCF-7 cells were infected with the indicated adenoviral vectors and/or treated with the indicated concentrations of purified bovine Cu/ZnSOD and/or catalase proteins prior to cytokine treatment and assessment of NF-κB transcriptional activity at 6 h post-cytokine treatment (mean ± standard error [SE]; n = 3). Paired comparisons (*, †) demonstrate significant differences (P < 0.05). Bottom panel of D: IKKα activity was assessed using immunoprecipitated IKKα and in vitro kinase assays with [γ-32P]ATP and GST-IκBα, followed by SDS-PAGE, transfer to nitrocellulose, autoradiography, and then Western blotting with anti-GST antibody. (B) Immunofluorescent localization of IL-1R1 in MCF-7 cells infected with Ad.Dyn(DN) (dominant-negative dynamin) or Ad.BglII (empty control vector) 48 h prior to treatment with IL-1β. Note that fixation conditions necessary for IL-1R1 staining lead to vesicle membrane breakdown and intracellular dispersal of signal. (C) MCF-7 cells were infected with Ad.Dyn(DN) (HA tagged) or Ad.BglII at various times prior to biotin-transferrin being added to the medium for 30 min. Cells were trypsinized, and cell lysates were evaluated by Western blotting with anti-HA antibody or avidin-horseradish peroxidase. (E) Loading of MCF-7 cells with various concentrations of purified bovine Cu/ZnSOD or catalase added to the medium for 20 min. Cells were trypsinized following treatment, and cell lysates were evaluated by Western blotting for uptake of enzymes. hSOD, endogenous human Cu/ZnSOD; bSOD, bovine Cu/ZnSOD. (F) MCF-7 cells were infected with the indicated adenoviral vectors 48 h prior to IL-1β (1 ng/ml) treatment in the presence of H2DCFDA (10 μM). Additionally, cells were simultaneously treated with 1 mg/ml bovine Cu/ZnSOD and catalase proteins at the time of H2DCFDA and IL-1β treatment. Control cells were not treated with IL-1β. Cells were washed and fixed at 20 min poststimulation and mounted in DAPI containing antifadent prior to fluorescence microscopy. Green DCF fluorescence is an indicator of H2O2 levels, and DAPI blue fluorescence marks nuclei.