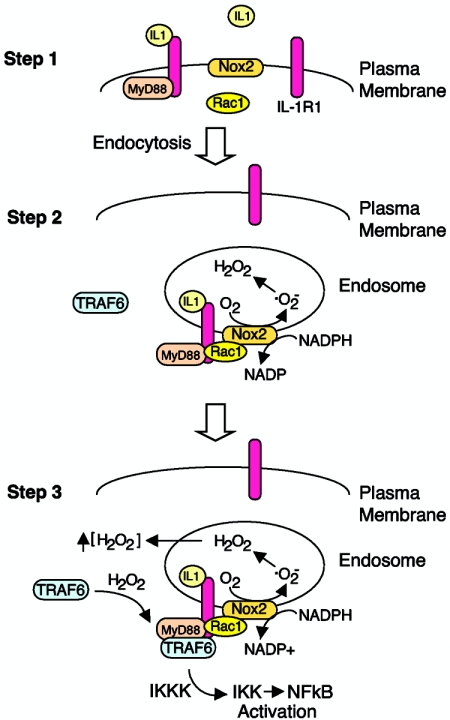
FIG. 8.
Summary of IL-1R1 receptor complex activation and its dependence on endosomal ROS. Schematically drawn is a model of three major steps in IL-1R1 complex formation following binding of IL-1β. (Step 1) Binding of IL-1β to IL-1R1 on the plasma membrane triggers MyD88 and Rac1 association with the receptor. Rac1 was required for the recruitment of Nox2 into the endosomal compartment, while MyD88 was required to initiate endocytosis of the receptor. Since IL-1β stimulation promotes ·O2− production in EEA1/Rab5-positive early endosomes (Fig. 4), our data support the hypothesis that Nox2 (an integral membrane protein) enters the endosomal compartment very early (i.e., from the plasma membrane). However, other scenarios are also possible, such as fusion of Nox2-containing vesicles with early endosomes containing ligand-activated IL-1R1. (Step 2) MyD88 association with IL-1R1 triggers endocytosis of MyD88/Rac1/Nox2, with IL-1R1 leading to NADPH-dependent ·O2− production by the endosomal compartment. Spontaneous dismutation of ·O2− to H2O2 likely gives rise to increased H2O2 both inside and outside the endosome, since H2O2 is permeable to membranes (2). (Step 3) Local increases in H2O2 facilitate the redox-dependent association of TRAF6 with the receptor complex on ligand-activated endosomes. The binding of TRAF6 to the receptor complex leads to activation of downstream IKK kinases (IKKK), IKK and, ultimately, NF-κB.